Abstract
BACKGROUND
Specific pulsed electromagnetic fields (PEMFs) have been shown to induce analgesia (antinociception) in snails, rodents and healthy human volunteers.
OBJECTIVE
The effect of specific PEMF exposure on pain and anxiety ratings was investigated in two patient populations.
DESIGN
A double-blind, randomized, placebo-controlled parallel design was used.
METHOD
The present study investigated the effects of an acute 30 min magnetic field exposure (less than or equal to 400 μTpk; less than 3 kHz) on pain (McGill Pain Questionnaire [MPQ], visual analogue scale [VAS]) and anxiety (VAS) ratings in female rheumatoid arthritis (RA) (n=13; mean age 52 years) and fibromyalgia (FM) patients (n=18; mean age 51 years) who received either the PEMF or sham exposure treatment.
RESULTS
A repeated measures analysis revealed a significant pre-post-testing by condition interaction for the MPQ Pain Rating Index total for the RA patients, F(1,11)=5.09, P<0.05, estimate of effect size = 0.32, power = 0.54. A significant pre-post-effect for the same variable was present for the FM patients, F(1,15=16.2, P<0.01, estimate of effect size = 0.52, power =0.96. Similar findings were found for MPQ subcomponents and the VAS (pain). There was no significant reduction in VAS anxiety ratings pre- to post-exposure for either the RA or FM patients.
CONCLUSION
These findings provide some initial support for the use of PEMF exposure in reducing pain in chronic pain populations and warrants continued investigation into the use of PEMF exposure for short-term pain relief.
Static, sinusoidal and low-frequency pulsed magnetic fields (PEMFs) have been shown to alter pain perception (nociception) and cognitive processing in both animals and humans (1–5). Our laboratory, and those of others, have demonstrated in snails (6), rodents (7), and humans (3) that single exposures to a sinusoidal, relatively weak PEMF tends to increase nociception. However, a single exposure to a specific low-frequency PEMF (8) can induce antinociception (ie, analgesia). To date, this has been observed in snails (5), rodents (9) and healthy volunteers (4,10). A single application of this PEMF has been shown to affect human electroencephalogram (2,11) and standing balance in both healthy humans (12) and patients with rheumatoid arthritis (RA) and fibromyalgia (FM) (13). The recent report (14) that a similar PEMF can reduce depression in patients with bipolar depression suggests that a PEMF can also influence affective state.
Taken together, these findings suggest that such weak PEMFs may alter pain perception in patients with chronic pain. We report here the effects of a 30 min exposure to a PEMF on pain levels in FM and RA patients using a double-blind, randomized, placebo-controlled parallel design.
PATIENTS AND METHODS
Participants
This study was approved by the Research Ethics Board for the Review of Health Sciences Research Involving Human Subjects at the University of Western Ontario, London, Ontario.
Thirteen female RA patients in study 1 (mean age 52.23 years, range 29 to 79 years) and 18 female FM patients in study 2 (mean age 51.28 years, range 35 to 67 years) were recruited from day treatment programs at St Joseph’s Health Care (London, Ontario). Participation in the program required a physician referral following a positive diagnosis for RA or FM by a rheumatologist (15,16). There were standardized criteria for chronic pain patients to be included in the program, which included pain history, diagnostic criteria and chronic pain level. The authors did not have access to the patients’ medical history, the population was not preselected in any way and selection bias was not applied. It was thought that this enrollment method provided the most robust and critical method for testing the treatment. Patients were narcotic free during the present study and were screened for depressive symptoms (concomitant depression was an exclusion criterion for the program). Subjects were numerically and randomly assigned on a computer-generated list and all blinding (data, equipment and exposure condition) was maintained by staff outside of the study.
Of the RA patients, seven were randomly assigned to the PEMF group (mean age 54 years, SD=15.87) and six were randomly assigned to the sham exposure group (mean age 50.71 years, SD=12.0). No patients withdrew from the study before completion of the study requirements. Nine of the FM patients were randomly assigned to each of the PEMF (mean age 51.5 years, SD=9.07) and sham (mean age 51 years, SD=9.90) exposure groups. One FM patient withdrew (sham group) before the exposure period due to feelings of anxiety unrelated to the research conditions.
Materials
All subjects were seated in a comfortable chair in a quiet room. A headset fitted with coils beneath the plastic ear coverings and connected by a wire to the portable PEMF generating unit was placed with the earpieces covering the patient’s temples. The headset covered the area that extended from above the temple to just above and behind the ear, on both sides of the head. Consequently, the treatment area was the area of the central nervous system that went from immediately above the temple to just above and behind the ear, extending from the outer periphery of the cingulate cortex to the brain midline. The PEMF unit was designed to have two pulse sequence patterns: one pattern was set to deliver a zero-amplitude magnetic field (MF) exposure (sham), while the other pattern produced a PEMF of a maximum of 200 μT (2 Gauss) to the deep brain and a maximum of 400 μT (4 Gauss) at the headset. The frequency content of the MF as determined by Fourier analysis was less than 1 kHz. The pulse design used in the current study is described in the United States patent #6,234,953 (8).
The MF was not physically detectable by either the experimenter or the participant. No sound or vibration was emitted and there were no visual indicators on the unit other than a blinded ‘a’ or ‘b’ switch setting for conditions. The experimenter was provided with a randomized and blinded schedule of the ‘a’ or ‘b’ switch settings before each run of sessions for a day.
The McGill Pain Questionnaire (MPQ) (17) was used to assess subjective measures of clinical pain both before and after the delivery of the PEMF or sham exposure. This questionnaire consisted of four major classes of word descriptors: sensory, affective, evaluative and miscellaneous. Patients were asked to select the most fitting word in each of the 20 categories that pertained to their current pain level. A category was omitted if none of the words were relevant to the patient’s pain. Words within each category were ranked in order of appearance; a sum of the selected words according to their ranking provided the clinician with a Pain Rating Index (PRI). In addition to the PRI, an overall Present Pain Intensity (PPI) measure was provided on the questionnaire. This question asked patients to indicate their level of current pain intensity on a six-point Likert scale, ranging from no pain (0) to excruciating pain (5). The MPQ has been successfully tested for reliability and validity (17).
Visual analogue scales (VAS) (18) were used to assess levels of pain and anxiety, both before (pre) and after (post) MF or sham exposure. The pain scales ranged from no pain to worst possible pain. The anxiety scale ranged from no anxiety to worst possible anxiety.
The Beck Depression Inventory-II (BDI-II; The Psychological Corporation, USA), the most widely used instrument for detecting depression, is consistent with diagnostic criteria listed in the Diagnostic and Statistical Manual of Mental Health Disorders-IV (19). This questionnaire was quick and easy to complete; it contained four to six sentences from which individuals were expected to select the one that best described their experiences over the previous two weeks. The BDI-II has been shown to provide reliable, internally consistent and valid scores in medical settings (20). This questionnaire was administered at the beginning of the study to verify the patients’ depression level.
Procedure
Patients were randomly assigned to either the sham (no PEMF exposure) or the PEMF exposure conditions. The purpose of the study was explained and informed consent was obtained from the patients before the beginning of the experiment.
Once seated comfortably in the chair, patients completed the MPQ, the VAS for both pain and anxiety and the BDI-II. Patients were also asked to report their handedness and when their last menstrual cycle ended. The headset was then secured on the patients’ temples. After 15 min of recording physiological data (heart rate and respiration), the PEMF device was set to deliver the random but blind condition. Following 30 min of PEMF or sham exposure, an additional 10 min of rest (with no exposure) was recorded after which the MPQ and the pain and anxiety VAS scales were completed a second time. Patients were left alone in the experiment room but were provided with a paging device to have access to the experimenter at any time. The specific settings for the sham and PEMF exposure were kept blind to both the patient and the experimenter, and the code was broken following all data collection. Participants were queried as to which condition they thought they had received and asked if they had anything else such as adverse events to report. Analysis indicated that the participants guessed their condition at a random rate (their guess was not significantly correlated to the actual condition).
All of the analyses were performed using SPSS version 11.0, (USA). Analyses were performed separately on each of these independent studies (study 1: RA patients; study 2: FM patients). Pre- versus post-exposure results (repeated measures) were tested a priori to account for possible confounding placebo effects in the sham exposure groups. Where interactions were not significant, particular attention was paid to alpha and estimate of effect size (eta2) values. Pain and anxiety data were analyzed using repeated measures ANOVA. Covariates (eg, age, handedness, menstrual cycle phase and depression rating) were analyzed and not found to change any of the significance levels reported below. All hypothesis tests used α=0.05.
RESULTS
Study 1: RA patients
Demographic information
There was no significant difference in age between patients randomly assigned to the two groups, t(11)= −0.43, P>0.1.
Pain ratings
A significant interaction was found between the pre-post pain rating and type of exposure, ie, the effect of pre-test versus post-test condition on pain ratings differed across the exposure conditions, with a large reduction of pain noted in the PEMF-exposed group and a lesser reduction in the sham exposed group. Table 1 displays the specific numbers and significance values for the overall and subcomponent parts (including the PPI) of the MPQ. Specifically, a repeated measures ANOVA revealed the significant pre-post × condition interaction for the MPQ PRI (Total), F(1,11)=5.09, P<0.05, partial eta2=0.32, power = 0.54. This was confirmed by t test due to the disparity in pre-exposure pain levels between the two groups (pre-score minus post-score tested between the sham and MF conditions [t=2.26, P<0.05]). There was also a significant main effect of pre-post testing, F(1,11)=37.51, P<0.01, partial eta2=0.77, power = 1.0.
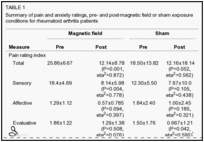
Similar findings were found for the miscellaneous subscale of the MPQ. Results from the sensory, affective and evaluative sub-scales, as well as the PPI of the MPQ, revealed significant main effects of pre-post testing; however, prepost testing × condition interactions were nonsignificant.
VAS – Pain
The only significant change using the VAS pain rating was found within the PEMF group: these patients had reduced pain ratings after the PEMF exposure. Conversely, PEMF versus sham exposure on the VAS pain rating did not differ between the pre-test and post-test times, and the effect of test time on its own did not lead to any changes in pain rating (Table 1).
Specifically, patients randomly assigned to the PEMF groups had significantly reduced pain ratings following their exposure period, F(1,6=7.84, P<0.05, partial eta2=0.57, power = 0.65; patients in the sham exposure group did not report significantly reduced VAS pain ratings, F(1,5)=0.05, P>0.10, partial eta2=0.01, power = 0.05. The pre-post-testing × condition (PEMF versus sham exposure) interaction for VAS pain ratings was nonsignificant, F(1,11)=3.95, P>0.10, partial eta2=0.26, power = 0.44. The main effect of pre-post-testing was also non-significant, F(1,11)=3.95, P>0.10, partial eta2=0.26, power = 0.44.
VAS – Anxiety
Table 1 displays the mean anxiety ratings reported by RA patients randomly assigned to the PEMF and sham exposure groups both pre- and post-exposure. Analysis of these results revealed a nonsignificant reduction in anxiety ratings, F(1,11)=1.64, P>0.10, partial eta2=0.13, power = 0.22. Furthermore, there was no significant condition by pre-post testing interaction for anxiety ratings, F(1,11)=1.45, P>0.10, partial eta2=0.12, power = 0.20.
Study 2: FM patients
Demographic information
There was no significant difference in age between patients randomly assigned to the two groups, t(15)=0.11, P>0.10.
Pain ratings
Using the MPQ, the only decreases in pain ratings were made by the subjects that were assigned to the PEMF group. Repeated measures ANOVA revealed a significant overall pre-post-effect for the MPQ PRI (Total), F(1,15=16.16, P<0.01, partial eta2=0.52, power = 0.96. The PEMF group, F(1,8)=17.60, P<0.01, partial eta2=0.69, power = 0.96, but not the sham group, F(1,7)=3.98, P=0.09, partial eta2=0.36, power = 0.41 showed a significant decrease in the overall pain rating following the exposure period. There was no significant interaction between pre-post-testing and condition (sham versus PEMF exposure) on this pain rating measure, F(1,15=0.32, P=0.58, partial eta2=0.02, power = 0.08.
Similar findings were found for the sensory, affective and evaluative subscales of the MPQ. Table 2 displays the specific numbers and significance values for the overall and component parts (including the PPI) of the questionnaire.

The miscellaneous subscale of the questionnaire did not yield the same results; there was no significant effect of pre-post-testing across groups (F[1,15]=2.19, P=0.16, partial eta2=0.13, power = 0.28), of pre-post-testing for the PEMF (F[1,8]=3.33, P=0.11, partial eta2=0.29, power = 0.36) or sham groups (F[1,7]=0.18, P=0.68, partial eta2=0.03, power = 0.07), or of pre-post-testing by condition interaction (F[1,15]=0.64, P=0.44, partial eta2=0.04, power = 0.12).
In contrast, there was a significant pre-post effect across groups (F[1,28]= 35.05, P=0.001, partial eta2=0.56, power = 1.00) for the PPI scores. These scores were significantly decreased pre- to post-exposure for both the PEMF-exposed group of patients (F[1,9]=18.00, P=0.003, partial eta2=0.69, power = 0.96) and the sham-exposed patients (F[1,7]=24.65, P=0.002, partial eta2=0.78, power = 0.99).
VAS – Pain
Using the pain ratings from the VAS, a significant decrease in pain ratings was found after both sham and PEMF exposure (Table 2). A significant pre-post exposure effect was noted for VAS pain ratings, F(1,13)=23.70, P<0.001, partial eta2=0.65, power = 1.00, with decreased pain scores present following the exposure period. Patients randomly assigned to both the PEMF and sham groups had significantly reduced pain ratings following their exposure period, F(1,7)=26.85, P<0.01, partial eta2=0.79, power = 0.99 and F(1,6=6.39, P<0.05, partial eta2=0.52, power = 0.56 for the PEMF and sham groups, respectively. No pre-post-testing by condition interaction existed.
VAS – Anxiety
Table 2 displays the average anxiety ratings reported by patients randomly assigned to the PEMF and sham exposure groups both pre- and post-exposure. Analysis of these results revealed a significant overall reduction in anxiety ratings across the entire patient pool, F(1,13)=5.21, P<0.05, partial eta2=0.29, power = 0.56; however, anxiety ratings did not significantly change across pre-post-testing for patients when analyzed separately by group, F(1,7)=2.83, P=0.14, partial eta2=0.29, power = 0.31 and F(1,6=2.59, P=0.16, partial eta2=0.30, power = 0.27 for the PEMF and sham groups, respectively. Furthermore, there was no significant condition by pre-post-testing interaction for anxiety ratings, F(1,13)=0.50, P=0.49, partial eta2=0.04, power = 0.10.
DISCUSSION
The results indicate that exposure to a specific low-frequency PEMF appears to have some beneficial analgesic properties, particularly in patients with RA. The results for the FM patient sample were mixed.
Pain ratings (MPQ and VAS)
Both the RA and FM patients randomly assigned to the sham and PEMF exposure groups reported decreased pain ratings following the 30 min trial period. Specifically, the RA patients exposed to the PEMF experienced a larger reduction in pain ratings than patients in the sham exposure group according to the pain rating on the MPQ (total) and VAS. For the FM patients, those in the PEMF group also had post-exposure pain ratings on the MPQ (total) that were more reduced by the exposure period (a priori hypothesis) compared with the control subjects; however, on the VAS, the FM patients who received both the PEMF and sham exposure showed a decrease in pain, with a greater decrease in the PEMF-exposed group. For RA patients, these findings were supported by the presence of a significant condition by time of testing interaction. Patients randomly assigned to the PEMF group had a significantly greater reduction in MPQ PRI scores than those in the sham exposure group.
All patients in the present study reported decreased pain ratings across time, an occurrence that can be attributed to the placebo effect. As defined by Kleinman et al (21), the placebo effect is the observation of a psychological or physiological change associated with inert treatments, sham procedures or therapeutic encounters. In the present study, patients were exposed to a therapeutic encounter: administration of either PEMF or sham exposure. For some of these patients, no treatment modality administered before participation in the current study, either pharmacological or non-pharmacological, was providing pain relief. The presence of a potentially effective and beneficial therapeutic treatment was likely encouraging to these patients; the potential benefit may have driven these patients to voluntarily participate in the study and expect a benefit.
The partial eta2 values obtained for patients in the two exposure groups (PEMF and sham) are consistent with the view that PEMF exposure confers a benefit greater than that obtained by expectancy or the placebo effect. For RA patients, the average partial eta2 value obtained for the pain ratings was 0.87 for patients in the PEMF group and 0.56 for the sham-exposed patients. Values for the FM patients were 0.69 and 0.36 for the PEMF and sham exposure groups, respectively.
Aside from the placebo effect, decreases in pain ratings for patients randomly assigned to the sham group can be attributed to relaxation. Staud et al (22) have reported that patients with FM report improvements in chronic pain following periods of rest. In the present study, the 55 min experimental period in which patients were seated in a comfortable chair could be considered a setting of relaxation; this time period of relaxation may have been the catalyst for reduced pain ratings post-exposure. Alternatively, activities in which the patients partook before enrollment and/or participation in the present study (eg, exercise training, household work), which were not controlled by the study administrators, may have exacerbated the patients’ pain symptoms (22), resulting in elevated pre-exposure pain ratings for patients in both the PEMF and sham exposure groups. Even if relaxation or prior activity participation were the cause of altered pain ratings, patients in the PEMF group benefited from significantly reduced pain ratings on a number of the tested scales (eg, PRI) post-exposure while patients in the sham exposure group did not.
Pain ratings assessed via the PPI and the VAS provided mixed results for both patient populations. Patients in the PEMF group for both patient populations reported significantly reduced VAS scores; however, of the sham-exposed patients, only the patients in the FM sample reported significantly reduced scores. For the PPI, significantly reduced scores were reported for RA patients in the sham exposure group and FM patients in the PEMF and sham exposure groups. PPI scores were not significantly reduced for RA patients in the PEMF group. One possible explanation for these results is that both the VAS and PPI refer to the intensity of the experienced pain in contrast to the quality of pain that is measured through the PRI. By memory alone, patients can improve their pain rating on the PPI. While both of the PPI and VAS measures have been shown to be valid and reliable indicators of subjective pain ratings, the finding that the MPQ had significantly reduced pain ratings only for the PEMF-exposed patients (and that a significant condition by time of testing interaction was present for RA patients for the MPQ) provides evidence, albeit mixed, of the analgesic conferring properties of exposure to the PEMF. Furthermore, it is a possibility that PEMF exposure influences the quality of experienced pain but not pain intensity itself.
Results of the present study are consistent with past research such that exposure to a specific PEMF has been shown to increase latencies on a hot plate in snail (5,23,24) and rodent models (9), as well as increase thermal (4) and electric (10) thresholds in humans. The effect of such extremely low-frequency PEMFs on pain and other behavioural responses is likely due to a direct sensing mechanism within tissues and cells (6). Finally, exposure to PEMFs has been studied for a large variety of clinical indications and has been shown to have encouraging results for most of the conditions studied (25).
CONCLUSION
Results of the current study confirm past findings in snails, rodents and humans exposed to acute pain that exposure to a specific PEMF has a modest pain-reducing effect in patients with RA. For these patients, exposure to a low-frequency PEMF produced decreases in pain beyond those found for a sham treatment control group. Interestingly, this effect was not found when FM patients were compared using an identical protocol. Future research using possibly more optimal PEMF parameters should be conducted to better understand how and when PEMFs produce reductions in clinical pain.
Acknowledgments
The authors thank Mr Lynn Keenliside for his technical assistance and equipment blinding, and Mr John Robertson and Ms Jennifer Hensel for their help with randomization and double-blinding. Supported in part by the Canadian Institutes of Health Research (FSP); the Natural Science and Engineering Research Council (NMS); Lawson Health Research Institute (AWT); Fralex Therapeutics Inc (AWT); the Ontario Research and Development Challenge Fund (AWT); the Canadian Foundation for Innovation (FSP); Ontario Innovation Trust (AWT, FSP); and St Joseph’s Health Care Foundation (AWT).

 EN
EN