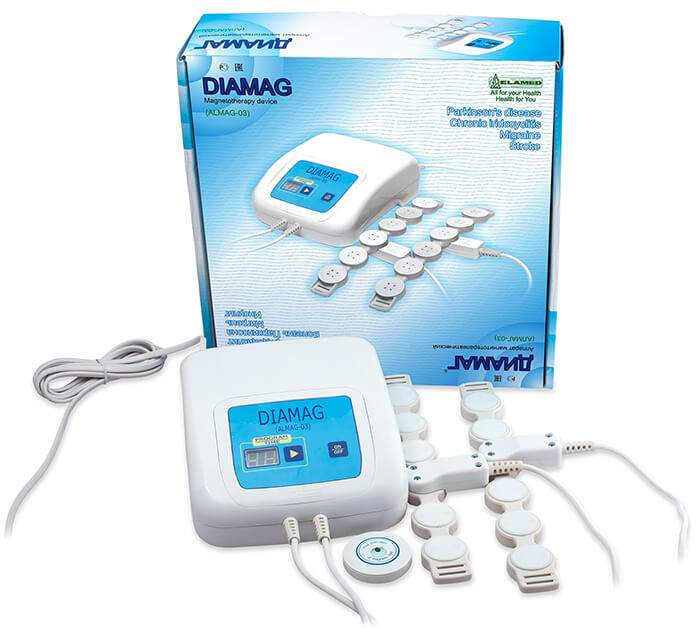
A Complete Guide About Allodynia Treatment
Allodynia is a type of pain related to nerves. After all, about 10 % of people in the US have some neuropathic pain. At the same time, about 15% to 20% of people with neuropathic pain have allodynia. People who are suffering from allodynia pain are extremely sensitive to touch. They even feel severe pain […]
A Complete Guide About Allodynia Treatment Read Post »