Clinical Experience Reveals Promising Results
Introduction: Why Is ALMAG-02 Widely Used in Clinical Rehabilitation?
Between 2014 and 2020, the Physiotherapy Department of City Hospital No. 37 (Nizhny Novgorod) conducted extensive clinical use of the ALMAG-02 PEMF therapy device, designed for treatment with low-frequency, low-intensity pulsed electromagnetic fields.
During this six-year period, three ALMAG-02 devices were used daily to treat 50–70 patients, integrating magnetic therapy into rehabilitation protocols for neurological, musculoskeletal, traumatic, respiratory, vascular, ENT, and gynecological conditions.
The accumulated clinical data demonstrates that ALMAG-02 significantly improves treatment outcomes, accelerates recovery, and is safe for long-term use, including in elderly and comorbid patients.
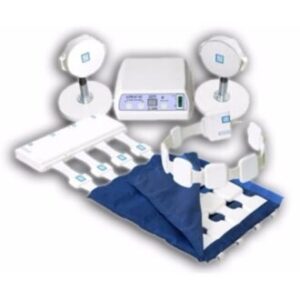
ALMAG-02 Device Overview
The ALMAG-02 is a modular PEMF therapy system engineered for local, zonal, and systemic treatment, allowing physicians to customize therapy based on pathology and anatomical region.
Technical Capabilities
| Feature | Description |
|---|---|
| Magnetic field types | Running and stationary |
| Programs | 79 preset therapeutic programs |
| Exposure time | 10–30 minutes |
| Penetration depth | Up to 15 cm |
| Application method | Contact |
| Tolerability | Excellent, including elderly patients |
| Safety | No endogenous heat, no adverse effects reported |
Emitter Configurations
| Emitter Type | Clinical Use |
|---|---|
| Main Emitter (ME) | Large zones (spine, chest, pelvis, limbs) |
| Separate Flexible Ruler (SFR) | Linear or circumferential areas |
| Local Emitters (LE) | Deep, localized pathology |
This flexibility allows simultaneous treatment of multiple zones, accelerating therapeutic response.
Clinical Applications and Outcomes
1. Peripheral Nervous System Disorders
Treated conditions included:
-
Femoral, sciatic, and peroneal neuropathies
-
Postherpetic neuropathy
Observed effects:
-
Anti-edematous and anti-inflammatory action
-
Pain reduction (VAS scale)
-
Improved microcirculation
-
Relief of muscle spasm
-
Improved motor function and gait
Patient Distribution
| Diagnosis | Patients | Age 30–50 | Age 51–70 |
|---|---|---|---|
| Peripheral neuropathies | 23 | 10 | 13 |
| Postherpetic neuropathy | 17 | 7 | 10 |
2. Musculoskeletal and Connective Tissue Disorders
ALMAG-02 was widely used in chronic and acute orthopedic conditions.
| Condition | Patients |
|---|---|
| Epicondylitis | 68 |
| Calcaneal spur | 76 |
| Tendovaginitis | 25 |
| Zudek’s syndrome | 15 |
| Vertebrogenic syndromes | 21 |
| Osteoporosis | 7 |
Example: Epicondylitis Treatment Protocol
| Parameter | Value |
|---|---|
| Program | No. 31 |
| Field type | Running |
| Induction | 20 mT |
| Frequency | 100 Hz |
| Exposure time | 15 minutes |
Outcome: Significant reduction in pain and inflammation, improved elbow function.
3. Zudek’s Syndrome (Acute Trophoneurotic Bone Atrophy)
ALMAG-02 demonstrated superior edema regression compared to standard therapy.
Edema Reduction Dynamics
| Day | Without ALMAG-02 (cm) | With ALMAG-02 (cm) |
|---|---|---|
| 0 | 27 | 27 |
| 5 | 26 | 27 |
| 10 | 25 | 26.5 |
| 15 | 23 | 25.5 |
| 20 | 22 | 25 |
Conclusion: ALMAG-02 improved microcirculation, reduced edema, and accelerated recovery.
4. Traumatic Injuries and Post-Surgical Recovery
ALMAG-02 was applied in:
-
Wounds after surgical debridement
-
Ligament injuries and dislocations
-
Fractures and contusions
-
Nerve injuries
Pain Regression Comparison
| Day | Control Group | ALMAG-02 Group |
|---|---|---|
| 5 | 17% | 40% |
| 10 | 41% | 65% |
| 15 | 65% | 84% |
| 20 | 80% | 100% |
Key findings:
-
Faster wound cleansing (2–3 days earlier)
-
Earlier epithelialization
-
Faster pain and edema reduction
-
Improved joint mobility
5. Respiratory Diseases
| Condition | Patients |
|---|---|
| Viral pneumonia | 25 |
| Bacterial pneumonia | 38 |
| Exudative pleurisy | 7 |
Results:
-
Faster resolution of inflammatory infiltrates
-
Reduced recovery time by 3–5 days
-
Improved lung drainage and oxygenation
6. ENT Disorders
| Condition | Patients |
|---|---|
| Chronic laryngitis | 48 |
| Eustachitis | 19 |
| Sensorineural hearing loss | 35 |
Clinical outcomes:
-
Reduced edema
-
Improved vocal function
-
Increased hearing acuity confirmed by audiometry
7. Circulatory and Cerebrovascular Diseases
| Condition | Patients |
|---|---|
| Dyscirculatory encephalopathy | 45 |
| Post-stroke conditions | 36 |
| Obliterating endarteritis | 18 |
| Chronic lymphedema | 6 |
Observed benefits:
-
Improved cerebral blood flow
-
Reduced dizziness and headaches
-
Stabilized blood pressure
-
Improved physical endurance and emotional state
8. Gynecological Disorders
| Condition | Patients |
|---|---|
| Salpingitis / oophoritis | 15 |
Results:
-
Reduced pelvic pain and congestion
-
Normalized menstrual cycle
-
Reduced adhesions (ultrasound)
-
Faster recovery (10–15 days earlier than control)
Safety and Tolerability
Across all applications:
-
No adverse effects observed
-
Well tolerated by elderly and comorbid patients
-
No thermal tissue effects
-
Suitable for repeated and long-term use
ALMAG-02 vs Standard Therapy
| Parameter | Standard Therapy | ALMAG-02 PEMF |
|---|---|---|
| Pain reduction | Gradual | Faster and more pronounced |
| Inflammation control | Medication-dependent | Drug-free |
| Recovery time | Longer | Shortened |
| Tissue regeneration | Limited | Enhanced |
| Side effects | Possible | None reported |
Conclusion
Extensive clinical use confirms that ALMAG-02 is a highly effective and safe medical-grade PEMF therapy device. When included in комплекс therapy and rehabilitation programs, it:
-
Accelerates healing and functional recovery
-
Enhances pain control and inflammation reduction
-
Improves outcomes across multiple disease groups
-
Shortens overall treatment duration
The results of real-world hospital use validate ALMAG-02 as a cornerstone technology in modern physiotherapy and rehabilitation medicine.
About Almagia International®
Almagia International® is the authorized U.S. distributor of ELAMED medical devices, including ALMAG-02. Since 2008, Almagia has provided FDA-registered, clinically validated non-invasive therapeutic technologies trusted by physicians and patients worldwide.
Disclaimer
This content is for educational purposes only and does not replace professional medical advice. Individual results may vary. ALMAG-02 should be used under medical supervision.
