Abstract
BACKGROUND
Benign prostatic hyperplasia (BPH) is a result of urogenital aging. Recent studies suggest that an age-related impairment of the blood supply to the lower urinary tract plays a role in the development of BPH and thus may be a contributing factor in the pathogenesis of BPH. The canine prostate is a model for understanding abnormal growth of the human prostate gland. We studied the efficacy of pulsed electromagnetic field therapy (PEMF) in dogs to modify prostate blood flow and evaluated its effect on BPH.
METHODS
PEMF (5 min, twice a day for 3 weeks) was performed on 20 dogs affected by BPH. Prostatic volume, Doppler assessment by ultrasonography, libido, semen quality, testosterone levels, and seminal plasma volume, composition and pH were evaluated before and after treatment.
RESULTS
The 3 weeks of PEMF produced a significant reduction in prostatic volume (average 57%) without any interference with semen quality, testosterone levels or libido. Doppler parameters showed a reduction of peripheral resistances and a progressive reduction throughout the trial of the systolic peak velocity, end-diastolic velocity, mean velocity, mean, and peak gradient of the blood flow in the dorsal branch of the prostatic artery. The pulsatility index and the resistance index did not vary significantly over time.
CONCLUSIONS
The efficacy of PEMF on BPH in dogs, with no side effects, suggests the suitability of this treatment in humans and supports the hypothesis that impairment of blood supply to the lower urinary tract may be a causative factor in the development of BPH. Prostate 74:1132–1141, 2014. © 2014 The Authors. The Prostate published by Wiley Periodicals, Inc.
INTRODUCTION
Benign prostatic hyperplasia (BPH) is an age-related enlargement of the prostate gland and is one of the most frequent medical disorders of elderly men throughout the world [1]. BPH also has a serious public health impact. Direct and indirect annual costs related to BPH treatment are estimated to be $3.9 billion in the United States [2–4] and €858 per patient per year in Europe [5]. BPH can present with hematuria, lower urinary tract symptoms and sexual dysfunction. If untreated, BPH leads to reduced quality of life and may result in severe complications such as acute urinary retention and urinary tract infections [6,7].
Generally the main goals of therapy for BPH are to improve symptoms, improve quality of life, arrest the disease process and prevent some of the adverse outcomes associated with BPH. The goals of BPH therapy may differ, however, depending on the individual’s point of view: patients focus on the quality-of-life issues and alleviation of symptoms and prefer a natural product with minimal side effects, particularly effects that impair sexual function or alter ejaculation; primary care physicians are concerned with safety of treatment used; and insurers and funders of national healthcare systems seek to minimize and defer treatment costs [8].
At the moment there are no therapies that meet all objectives. The available therapeutic options are surgical or pharmaceutical, each with pros and cons. Transurethral resection of the prostate (TURP) has been the gold-standard treatment in men with symptomatic BPH. However, the morbidity of TURP approaches 20%, and less invasive techniques being developed are reducing the need for this method [9]. Available drug therapies for BPH fall under two general categories: alpha-blockers and 5 alpha-reductase inhibitors, both of which can improve urine flow, but they do not reduce the size of the prostate, and can contribute to sexual dysfunction or hypotension as side effects.
Phytotherapies or dietary supplements (saw palmetto, pygeum africanum, etc.) are widely available and commonly used remedies, with evidence of efficacy, but may not have the quality and safety profiles of medicines regulated by the Food and Drug Administration and may have variable potency and pharmacological activity [10].
Each new therapeutic approach should not ignore BPH pathophysiology, even if not completely understood [11]. BPH is a disease with multiple etiologies, including hormone signaling, disruption of proliferation, and apoptosis dynamics, and chronic inflammation, with changes in the morphology and phenotype of the prostate stroma. Inflammation of the prostate represents a mechanism for hyperplastic changes to occur in the prostate. Both chronic and acute inflammation may lead to events that can cause proliferation within prostatic tissue through oxidative stress. Both tissue damage and oxidative stress may lead to compensatory cellular proliferation with resulting hyperplastic growth [12].
Systemic conditions such as hypercholesterolemia [13], obesity [14] and stress [15,16] may be important risk factors for BPH. Several studies suggested an association between prostatic disease and the presence of vascular disorders such as coronary heart disease [17,18] or diabetes mellitus [19,20]. Generalized or localized vascular damage may cause hypoxia. Ghafar and colleagues [1] postulated that hyperplasia in the stromal and glandular compartments of the prostate might be induced by stromal growth secondary to hypoxia, which in turn results from abnormal blood flow patterns. In a recent study using a cell-culture model of human prostatic stromal cells, the cells responded to hypoxia by up-regulating the secretion of several growth factors in vitro, which suggests that hypoxia might trigger prostatic growth [21].
These findings led our team to consider a new therapeutic option for BPH: pulsed electromagnetic field therapy (PEMF). We focused our attention on the following main concepts: (1) the prostatic vascular system is an important component of prostate growth and regulation, and the dysfunction in blood flow to the prostate gland may be involved in the process through which BPH develops and is controlled [1]; (2) inflammation represents a mechanism for hyperplastic changes to occur in the prostate [12]; and (3) PEMF has a positive effect on vascularization and hemodynamics of the prostate [22] and tissues in general [23]. Thus, reducing tissue hypoxia that results from abnormal blood flow patterns by improving oxygen delivery and reducing inflammation might be a healing measure or a preventive measure in patients with or at risk of BPH.
Nevertheless, there is some confusion about PEMF. In general, electromagnetic modalities include any modality which uses electricity and therefore generates both an electric field and a magnetic field. This includes PEMFs, microcurrent therapy (MCT) and microwave diathermy (MWD).
Ultrasound is a commonly used modality in which the resultant acoustic wave is mechanical and not electromagnetic [24]. Many other energy delivery system used to treat BPH [including transurethral electrovaporization of the prostate (TUVP), transurethral electrovapor resection of the prostate (TUVRP), transurethral electrovaporization of the prostate using bipolar energy (plasmakinetic vaporization of the prostate [PKVP]), visual laser ablation of the prostate (VLAP), transurethral microwave thermotherapy (TUMT)] have been developed as alternatives to surgical transurethral prostatectomy (TURP) [25].
PEMF is very low frequency pulsed energy waves in the range of 1–50 Hz and is also identified as a weak non-thermal electromagnetic field [26] generally used to expedite recovery [24] or to reduce post surgical side effects [27,28].
It is important to distinguish magnetostatic therapies (with natural stones) with electromagnetic ones. Magnetostatic therapies are based on the application of a motionless magnetic field.
Electromagnetic therapies are based on the application of time-varying magnetic fields, usually generated at low frequencies by an alternating current passing through a coil [29]. The essential difference with static fields is that time-varying magnetic fields can generate electric fields with significant intensity inside the body, and its value may be estimated using physical laws like the Faraday’s equation. Magnetostatic fields do not have an associated electric field and cannot transfer magnetic energy to moving charged particles, indicating the ineffectiveness of this treatment and explaining the absence of firm medical evidence in the scientific literature to support its use [29]. In this article, we use the term magnetotherapy or PEMF to refer to electromagnetic therapy by PEMF, where there is always an electrical field that influences the body’s electrical charges.
Over the past several decades, physicians and scientists have used a rigorous scientific approach to clinical application of PEMF to treat therapeutically resistant problems, mainly in the musculoskeletal system. PEMF has been proven to be clinically safe, and it is well accepted that PEMF provides a practical non-invasive method for inducing cell and tissue modification that can correct selected pathological states. Many publications suggest that exogenous electromagnetic fields can have profound effects on a large number of biological processes [30–33].
PEMF is a relatively widespread method used in several medical disciplines such as orthopedics, neurology, and urology [34]. The mechanism for the advantageous action of the pulsed magnetic field on the living organism is not quite clear yet, but clinical investigations have revealed a favorable anti-inflammatory, angioedematous, and analgesic therapeutic effect [35]. A number of clinical studies suggest that magnetic stimulation can accelerate the healing process [27,32,33,36]. Its use on the prostate gland led to a decrease in the number of postoperative complications after TURP [28] and was found to stimulate prostate glands and improve circulation [23], having a positive effect on vascularization and hemodynamics of the prostate [22].
The objective of the current study was to evaluate the efficacy of magnetotherapy for the treatment of BPH as a conservative therapy with high compliance, no drug interactions and low cost. As a preliminary study, the dog was used as experimental model. The dog is considered a reliable animal model for the study of human BPH [11,37–39] and few differences are noticeable between the two species in terms of prostatic anatomy [40], histology [41,42], physiology, BPH pathogenesis [43–45], diagnosis [46] and symptoms.
The most common clinical sign of BPH in dogs is bloody fluid dripping from the penis unassociated with urination. Although urethral obstructive disease occurs in men with BPH, it is extremely rare in dogs [47]. While blood is frequently observed in semen samples and the volume of ejaculate is decreased [43], total sperm count and fertility are not affected by BPH in dogs. However, most cases of BPH in dogs do not evidence clinical signs despite being viewable on ultrasonography as prostatic volume enlargement. It is only when the prostate becomes large enough to compress the colon and interferes with defecation that serious effects result and rectal tenesmus and constipation may be present [48]. Despite the differences, there are sufficient similarities between the two species to regard the canine condition as a useful model for comparison to the human disease [38,43].
In this study, we evaluated the effect of PEMF on prostate volume, semen quality, testosterone and hemodynamic changes by ultrasonography.
MATERIALS AND METHODS
Animals
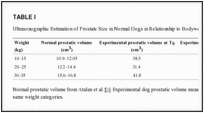
For the study, 20 mature, healthy male dogs 8 to 14 years of age (mean = 9.5 years, SD = 1.5 years), weighing 13–39 kilograms (kg) (mean = 23.8 kg, SD = 8.8 kg) were selected. No symptoms of prostatic disease were detected in experimental dogs apart from a uniform prostatic enlargement at rectal exploration and an increase of prostatic volume at ultrasonography that suggested diagnosis of simple BPH [43].
Cytological evaluation by fine-needle aspiration biopsy of the prostate confirmed the benign nature of the prostate volume enlargement [49].
Urinalysis and an andrological examination (including ultrasonography exams, semen quality, and prostatic fluid evaluation) were also performed. The selected dogs were all owned and not subjected to changes in habits during the study.
Clinical exams were performed at the Faculty of Veterinary Medicine, University of Bari Aldo Moro. Investigations were conducted with the owners’ consent in accordance with the Principles for the Care and Use of Research Animals, promulgated by the European Community.
Experiment
At day 0 (T0), the prostate gland was scanned by transabdominal ultrasonography and volume was calculated. Prostatic hemodynamic patterns were checked by Doppler. Semen collection and blood samples were performed to check semen quality, seminal plasma volume, and serum testosterone concentration.
Dogs were then treated two times a day (every 12 hours) with magnetotherapy for 5 min each session for three weeks. At day 7, 14, and 21 (T1, T2, T3) the prostatic volume and hemodynamic changes were checked by ultrasonography.
At day 0 and 21 (T0, T3), semen was evaluated for quality (total sperm concentration, motility), seminal plasma contents, volume and pH; blood samples were evaluated for testosterone content. Throughout the trial, the dogs were under clinical observation.
Magnetotherapy
Magnetotherapy was performed by a physical therapy device (Magcell® Vetri, Physiomed Elektromedizin AG, Schnaittach, Germany) (Fig. 1).

The PEMF can be identified as follows.
A frequency of 4–12 Hz and intensity of 1000 Gauss was used. The magnetic field maximum strength was 200 mT. This can be explained by considering that ±105 mT (approximately 1,000 Gauss) is the amplitude of flux density measured at the magnetically active surface of the Magcell® when the unit is switched on. This results in 210 mT (approximately 2,000 Gauss) peak to minimum of the sinusoidal wave (measured by Kernforschungszentrum Karlsruhe GmbH, Institut für Toxikologie, Karlsruhe, Germany). This was an AC field based on sinusoidal pulses with frequencies between 4 and 12 Hz with 8 Hz (mean frequency of therapy program) with the pulse rate of 12.5 ms. The electromagnetic field was used in continuous mode. Intensity in the various tissues was calculated with the formula i = κ · π · f · r · B.
[i = induced current: κ = specific tissue conductance (S/m; S for Siemens); π = figure Pi (3.14); f = frequency of magnetic field (Hz); B = flux density of magnetic field (T) and r = radius of magnetic source respective distance from center line (m)].
The following values were set on the Magcell®: f = 8 Hz (mean frequency of therapy program); r = 0.03 m (radius of rotating magnet fisk); B = 0.105 T (flux density on surface Magcell®/tissue surface). The field intensity was reported as half-wave.
The device was pressed against the dog’s skin corresponding to the inguinal region where the prostate is located (Fig. 2). Therapy started when the device was switched on and automatically switched off 5 min later.

Ultrasonography
Dogs with prostatic hyperplasia were selected for the study by using ultrasonography (Sonoace Pico Medison-Korea) to identify dogs with symmetric, mild enlargement of the gland with mildly increased echogenicity. After basic prostatic dimensions were obtained, the size of prostate gland was calculated from the equation: volume (cm3) = [(L + W + D)/2.6] + 1.8 where: L (length) = cranio-caudal diameter; W (width) = transversal latero-lateral diameter; D (depth) = dorso-ventral diameter [50]. Prostatic volume was higher in the subjects when compared with normal canine prostatic size [51] (Table (TableII).
Using color-Doppler (7.5 MHz micro-convex probe, Doppler frequency 4 MHz, sample volume of 2–4 mm), five parameters were examined at T0–T3: the systolic peak velocity (SPV), end-diastolic velocity (EDV), mean velocity (MV) of the blood flow in the dorsal branch of the prostatic artery mean and peak gradient (G). By using previous parameters, pulsatility index (PI) and resistive index (RI) were calculated according the formulas of Gosling (SPV-EDV)/MV and Pourcelot (SPV-EDV)/SPV, respectively [52].
Semen Quality and Prostatic Fluid
At T0 and T3 semen was collected by digital manipulation of the penis [53], using disposable semen collection cones (artificial vaginas) (IMV Technologies, S.r.l., Piacenza, Italy) and graded tubes. The ejaculate volumes were measured and recorded.
In the dog the ejaculate is typically collected in three fractions. The second sperm-rich fraction of the ejaculate was examined by computer-assisted sperm analysis (CASA) (IVOS Version 12.2; Hamilton Thorne Biosciences Inc., Beverly, MA) for total sperm concentration and percentage of motile sperm [54].
The third fraction is solely prostatic fluid. Since the dog possesses neither seminal vesicles nor bulbourethral glands, the prostatic contribution to the total fluid volume of semen is >90% [55]. The volume of the third fraction is the most variable and can exceed 15 ml in normal dogs [56].
Third fraction volume contents were used to assess prostatic secretory function and the prostatic fluid was microscopically checked for abnormalities (e.g., red blood cells). Prostatic fluid pH was also estimated. It was measured with a pH meter (Consort C830, Consort Electrophoresis power supplies, Turnhout, Belgium). Before semen pH measurements, the pH electrode was previously stored in KCl solution, then rinsed in a beaker containing distilled water, after which the electrode was immersed in a container that contained seminal plasma and the pH value was recorded.
Assay for Serum Testosterone
Blood was sampled by vein-puncture at T0 and T3. Testosterone was measured considering the physiological secretory patterns. All collections were made between 9:30 and 11:30 a.m. The blood collected was immediately centrifuged (1500g, 15 min at room temperature) so the serum could be separated and stored at −20°C until assay. Testosterone was measured by a chemiluminescence technique (Immulite Immunoassay System, Siemens, Healthcare Diagnostics S.r.l., Milano, Italy).
Routine Clinical Observation
All animals were subjected to routine clinical observation during the T0–T3 period. During clinical observation, parameters considered included body weight, blood counts, general attitude, appetite, rectal temperature, prostate palpation, heart, and respiratory rates.
Statistical Analysis
All data were summarized for each individual animal by parameter measured (age, weight, testosterone level, total sperm count, sperm motility, seminal plasma volume and pH, Doppler measurements, and prostatic volume), and time point (T0–T3) using the Microsoft Excel 2011 program (Microsoft Corporation, Redmond, WA). These data were described in terms of the average and standard deviation (SD) (mean ± SD) in the text for brevity.
Statistical analyses were conducted using Statistica (StatSoft, Inc. Tulsa, OK). Data were first evaluated for normality and to determine if there was a relationship between age or weight of the dog and the dependent variables or the treatment effect. For normally distributed data, a repeated measures design (dependent variable t-test) was used to evaluate the data, comparing the baseline measure to the follow-up measure(s) for prostate volume, seminal plasma volume, seminal plasma pH, total sperm count, sperm motility and testosterone level. Two tailed tests with P < 0.05 were considered as significant. For Doppler data variables (SVP, EVP, MV, mean and peak G, RI and PI) the nonparametric repeated measures test Friedman’s ANOVA was used due to violations of assumptions. Additionally, the Bonferroni adjustment was applied due to repeated tests on these data variables, resulting in significance defined as P < 0.007.
RESULTS
During the experiment, dogs did not showed signs of discomfort. No local or systemic adverse effect was noticed. During PEMF, dogs remained calm. No variations were noticed in body weight, blood counts, urinalysis, rectal temperature, urethral blood discharge, heart and respiratory rates, general attitude during PEMF and through the clinical trial. At all times libido was conserved and semen collection was easily performed.
Prostatic Volume
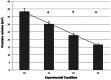
As shown in Figure 3, a progressive reduction of prostatic volume was recorded from T0 to T3. Prostate volume decreased an average of 57% over the course of the study, from 38.61 ± 9.3 to 16.62 ± 2.4 cm3. Older dogs had marginally smaller prostatic volume at T1 and T2 (r = −0.47, P < 0.035), but there was no correlation between age and change in prostate volume. Prostatic volume was significantly decreased at each of the timepoints T1–T3 as compared with baseline T0 [T0 vs. T1: t (1,19) = 6.70, P < 0.001; T0 vs. T2: t (1,19) = 11.02, P < 0.001; T0 vs. T3: t (1,19) = 12.80, P < 0.001].
Color-Doppler
Spectral analysis of Doppler parameters (SPV, EDV, MV, mean, and peak G) (Table (TableII)II) showed the reduction of peripheral resistances and a progressive reduction of SPV and EDV from T1 to T3. The Friedman’s ANOVA test was significant for SPV [χ2(3) = 60.0, P < 0.0001] and EDV [χ2(3) = 53.0, P < 0.0001], with measurements at baseline significantly higher than all timepoints after treatment (see Table TableII).II). Similarly, analysis revealed significant differences for MV [χ2(3) = 51.7, P < 0.0001], mean G [χ2(3) = 44.3,P < 0.0001] and peak G [χ2(3) = 37.4, P < 0.0001]. The pulsatility index (PI) and the resistance index (RI) did not vary significantly over time [PI: χ2(3) = 1.98, P = 0.58; RI: χ2(3) = 4.80, P = 0.187].
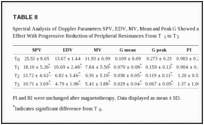
Age of the dog was not related to Doppler measurements, but for many measurements weight was significantly positively related (P < 0.05) (PSV ave. r = 0.69; MV ave. r = 0.74; G mean ave. r = 0.69; G peak ave. r = 0.52). In addition, the treatment effect from baseline to T3 was significantly positively correlated with weight for PSV (r = 0.46, P < 0.044), G mean (r = −0.46, P < 0.040) and G peak (r = −0.55,P < 0.011).
Semen Quality and Prostatic Fluid
Semen characteristics were conserved over the treatment period. At T0 versus T3 the mean total sperm count (×106) was 438.4 ± 43.6 versus 443.7 ± 38.8 and the difference was not significant [t(1,19) = −1.49, P = 0.153]. The percentage of motile sperm was 86.6 ± 5.3 versus 87 ± 4.3 at T0 versus T3. The difference was not statistically significant [t(1,19) = −0.67, P = 0.508].
Seminal plasma volume, which reflects mainly prostatic fluid volume, did not significantly change over the course of the study [t(1,19) = −1.14, P = 0.27]. At T0, the mean volume was 4.63 ± 0.88 ml and at T3 it was 4.72 ± 0.89 ml. As expected, there was a significant positive relationship between weight of the dog and seminal plasma volume (T0: r = 0.82, P < 0.001; T3: r =.80, P < 0.001). However, the subjects’ weight was not related to treatment effect (weight and the change in volume from T0 to T3: r =.03, P = 0.9).
No significant difference in seminal plasma pH level from baseline (6.28 ± 0.14) to study conclusion (6.27 ± 0.13) was noted [t(1,19) = 0.14, P = 0.89].
Testosterone
Testosterone levels were at physiological range throughout the study. There was no significant difference in testosterone at T0 (634 ± 193 ng/dl) and at T3 (638 ± 196 ng/dl) [t(1,19) = −0.71, P = 0.49].
DISCUSSION
Results from our study indicate that the electromagnetic treatment produced a significant reduction (average 57%) in prostatic volume in three weeks of treatment without any interference with semen quality or libido or general health status.
Seminal plasma composition, volume and pH, as well as sperm motility and testosterone levels, did not change over the course of the study.
Normally, prostatic secretory function declines with age or when histologic changes of BPH become more evident, as in dogs with complex BPH [43]; therefore an increase in seminal plasma is expected after treatment. In our study an increase in the seminal plasma volume after treatment did not occur. This can be explained by the fact that the selected dogs had simple BPH and were asymptomatic. This means that prostate enlargement was not great enough to produce symptoms such as an initial lower seminal plasma volume, which is a common situation in the dog. Although almost all intact male dogs develop BPH, with >95% affected by 9 years of age, most will not develop clinical signs associated with this condition [57].
We found no evidence of adverse effects of PEMF on reproductive function, consistent with other investigators’ previously reported results [58]. This study also confirmed the lack of impact of PEMF on hormonal balance, as no change in testosterone levels was found. Previous studies also reported no or very subtle effect on FSH, LH, prolactin, estradiol, and testosterone concentrations [59].
The spectral analysis of the different Doppler parameters (SPV, EDV, MV, PI, RI) highlights a reduction in peripheral resistance and a progressive reduction of systolic peak velocity and end-diastolic velocity from T1to T3. These data support the hypothesis that a dysfunction in blood flow to the prostate gland may be involved in the process through which BPH develops [1]. By producing an increase in blood circulation [28,60], PEMF may reduce the peripheral resistance and thus may help to prevent secondary complications caused by reduced arterial blood flow such as prostatitis [23]. These conclusions can also be supported by the recent finding [61] that erectile dysfunction drugs may cause small blood vessels in the prostate to dilate, improving BPH symptoms. Similar results were found after prostatic surgery [28]: PEMF improves hemodynamics in the prostate and decreases the number of postoperative complications.
The mechanism of action of PEMF on the canine process could involve several modalities. Some electromagnetic therapies involve a local heating effect since they use electromagnetic energy. For example, microwave is based on electromagnatic radiation and produces heat by conversion or absorption in the tissue. Shortwave therapy uses a PEMF at a high frequency in which heat is produced at induction of electric or magnetic turbulent fields in well-conducting tissues. However, there should be no tissue heating due to PEMF treatment itself and use of the Magcell® for PEMF, which utilizes an extremely low frequency, is physically athermal [26]. Because PEMF has a blood flow stimulating effect [28,60], there may be a minimal increase of local temperature as would normally occur in treated areas with poor perfusion. Any increase of local temperature is not directly caused by PEMF but is the result of the increase of blood flow. However, a localized heating effect is unlikely to explain the influence of PEMF on BPH in the dog.
Nitric oxide (NO) secretion may be a more likely mechanism of action of PEMF for BPH, as it is a strong promoter of microvessel dilatation in this context. Recent research demonstrates that PEMF stimulatory effects were mediated by the increase in NO synthesis [62].
In the canine prostate, NO release relates to growth and pathology. Low levels of neuronal nitric oxide synthase (NOS) expression in BPH tissue, compared with higher levels in atrophic tissue, suggest that neuronal NOS expression is down-regulated in the prostate with benign cellular proliferation whereas it is maintained or possibly up-regulated in the prostate with prostatic involution. Therefore, altered NOS expression contributes to the pathogeneses of BPH [63].
The research to date has shown that the mechanisms by which PEMF works are complicated and likely involve many pathways. Additional studies are needed to precisely determine the mechanism for these effects in BPH.
The anti-inflammatory function of electromagnetic-fields [34] should also be considered. As recently stated [12], prostatic inflammation may represent an important factor in influencing prostatic growth and progression of symptoms. The inflammation of the prostate may represent a mechanism for hyperplastic changes to occur in the prostate. There are a variety of growth factors and cytokines that may lead to an inflammatory process within the prostate.
PEMF’s greatest power was demonstrated in its ability to ameliorate the effects of inflammation by decreasing inflammatory cytokines in addition to increasing cell metabolism [24]. Therefore PEMF, by reducing inflammatory processes, may have a role in reducing changes linked to BPH and related symptoms.
CONCLUSIONS
This preliminary study investigated for the first time the effect of PEMF therapy on canine prostate glands affected by BPH. The results demonstrate the safety and efficacy of this therapy in reducing prostatic volume without negative impacts on libido or seminal parameters. Although the mechanism of action of PEMF therapy is not completely clear, the results of this study show a possible improvement in blood flow, supporting the theory that BPH might be caused by blood flow impairment.
The efficacy of PEMF therapy on dogs, used as an experimental model of BPH, suggests the suitability of this non-invasive treatment in humans. PEMF therapy represents a promising treatment for BPH given the minimal number of contraindications [35], lack of side effects, and affordable cost. It offers the ability to treat the underlying pathology rather than simply the symptoms. The time is particularly opportune to consider alternatives given the increased incidence of side effects from the use of pharmacological agents. PEMF therapeutics for BPH could have a profound impact upon health and wellness and treatment costs worldwide.
Nevertheless, further investigations need to consider the anatomical characteristics of the human prostate that differ from the canine model and investigate the efficacy on BPH in men.
Acknowledgments
This work was supported by Parsemus Foundation. The authors are deeply grateful to Parsemus Foundation, Berkeley, California, U.S.A. for financial assistance, continuous support, and interest in this study. Also, the authors sincerely acknowledge Linda Brent for analysis and interpretation of data and language revision. The authors are also grateful to Physiomed Elektromedizin AG, Germany for supplying Magcell® Vetri and for technical support.

 EN
EN