Abstract
Objectives: Direct current, capacitive coupling, and inductive coupling are modes of electrical stimulation (ES) used to enhance bone healing. It is important to assess the effectiveness of ES for bone healing to ensure optimization for clinical practice. This review aims to examine the level of evidence (LOE) for the application of ES to enhance bone healing and investigate the proposed mechanism for its stimulatory effect.Methods: MEDLINE and EMBASE searches were conducted to identify clinical and in vitro studies utilizing ES for bone healing since 1959. A total of 105 clinical studies and 35 in vitro studies were evaluated. Clinical studies were assigned LOE according to Oxford Centre for Evidence Based Medicine (LOE-1, highest; LOE-5, lowest). Results: Direct current was found to be effective in enhancing bone healing in spinal fusion but only LOE-4 supported its use for nonunions. Eleven studies were retrieved for capacitive coupling with LOE-1 demonstrating its effectiveness for treating nonunions. The majority of studies utilized inductive coupling with LOE-1 supporting its application for healing osteotomies and nonunions. In vitro studies demonstrate that ES enhances bone healing by changes in growth factors and transmembrane signaling although no clear mechanism has been defined. Conclusion: Overall, the studies, although in favor of ES application in bone repair, displayed variability in treatment regime, primary outcome measures, follow-up times, and study design, making critical evaluation and assessment difficult. Electrical stimulation shows promise in enhancement of bone healing; however, better-designed clinical studies will enable the optimization for clinical practice.
When bone encounters injury, it undergoes a unique process of self-regeneration to form new bone to heal itself. However, in 5% to 10% of patients this process is disrupted which leads to delayed bony healing or nonunions.1 This is of great consequence to the clinician as nonunions pose a huge burden on the individual in terms of continuing pain and disruption to their daily activities and increases the expenditure of medical resources. Therefore, finding effective methods to enhance bone healing has been of great research interest, one of which is the use of electrical stimulation (ES).
In the early 1950s, Fukada and Yasuda2 demonstrated that when stress is applied to bone in such a way to cause deformity electrical potentials are generated, in areas of compression the bone was electronegative and caused bone resorption, whereas areas under tension were electropositive and produced bone. Therefore, subsequent developments were based on the idea that stimulating these endogenous electric fields using an ES device would enhance bone healing.3
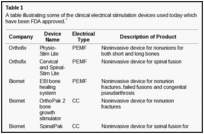
There are 3 methods of administering electrical current to bone (Fig (Fig1),1), which have been used in clinical practice (Table (Table1)1) including direct current (DC), capacitive coupling (CC), and inductive coupling (IC). In several models, DC involves invasive surgical placement of electrodes.1 A cathode is placed at the site of the bone defect with an anode in the soft tissue nearby.3 Osteogenesis has been shown to be stimulated at the cathode using currents between 5 and 100 µA and varying the number of electrodes between 2 and 4.3 Since the stimulator is implanted, the therapeutic treatment is continuous but is removed once healing has occurred. Direct current is advantageous as patient compliance is minimal; however, the technique is invasive with risk of infection, tissue reaction, and soft tissue discomfort.4
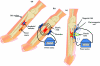
Capacitive coupling involves noninvasive placement of 2 cutaneous electrodes on opposite sides of the bone to be stimulated.3 A power source, usually attached to the patients cast is then connected to the electrodes forming an electrical field within the fracture site. Using potentials of 1 to 10 V at frequencies between 20 and 200 kHz creates electric fields of 1 to 100 mV/cm, which has shown to be efficient for bone stimulation.5
Inductive coupling enhances bone healing by using pulsed electromagnetic field (PEMF) stimulation. Inductive coupling is formed by placing 1 or 2 current-carrying coils on the skin over the fracture site.4 As current flows through the coils, an electromagnetic field radiates at right angles to the coil base but within the fractures site.4 The electrical field that is formed varies in size because of the type of tissues at the fracture site and the properties of the applied magnetic field.5 Electromagnetic fields varying from 0.1 to 20 G have been used to create an electrical field at the fracture site of 1 to 100 mV/cm.6 Inductive coupling and CC are beneficial treatment options for patients as they are noninvasive, painless, and surgery free.4 Furthermore, they can be easily and conveniently used by patients at home and in most cases patients are allowed to bear weight.4
Electrical stimulation has shown to be effective in aiding bone healing in a variety of orthopedic conditions such as aiding internal and external fixation,7 enhancing delayed or nonunion fractures8 and osteotomies,9improving the efficacy of bone grafts,10 treating fresh fractures,11 and aiding femoral osteonecrosis.12However, the mechanism by which ES has its stimulatory effect in enhancing bone healing remains unclear.3
Therefore, we performed a systematic review to address (1) what is the proposed mechanism of action for DC, IC, and CC (2) what is the level of evidence (LOE) supporting the use of DC, CC, and IC in enhancing bone healing for orthopedic conditions.
MATERIALS AND METHODS
An electronic search of the MEDLINE through PubMed and EMBASE databases was performed to identity all relevant clinical studies that utilized ES for the treatment of bone healing from 1959 to 2009 by 2 independent reviewers (M.G., A.B.). Over the same time period, all in vitro studies that assessed the mechanism behind ES were identified. Keywords with Boolean operators used in the search included the following: “bone healing” or “nonunion” or “fracture healing” or “fracture ununited” and ES or electrical therapy or electromagnetic field stimulation or pulsed electromagnetic field stimulation. Articles were considered eligible if included the following inclusion criteria: (1) inclusion of a treatment arm receiving ES of DC, CC, or PEMF to impact bone healing; (2) evaluated the use of ES treatment for long bone and non–long bone healing (spine, scaphoid, and clavicle); (3) evaluated the use of ES to impact bone healing including the effect of ES on enhancing nonunion or malunion or delayed union, spinal fusion, pseudoarthrosis, osteotomies, fresh fractures, and femoral osteonecrosis; and (4) in vitro studies that evaluated the mechanism behind DC, CC, or PEMF. Case reports and expert opinions were included to that all related studies were identified and reviewed. Articles were excluded if they were (1) not published in English, as the reviewers would not fully understand the manuscript; (2) animal studies as these reports only show the end result whether there has been an increase or decrease in bone development and do not give details for the mechanism of ES; (3) mode of ES other than DC, CC, or IC; and (4) review papers, editorials, publications on congress meetings, unpublished data, or letters to the editor. Review articles were only used to identify any other relevant articles.
Clinical studies were then grouped by the primary method of ES used (DC, CC, or IC) and then assessed and assigned an LOE adapted from the Oxford Centre for Evidence Based Medicine (http://www.cebm.net/index.aspx?o=1025) to establish whether valid and reliable evidence supports the use of ES for bone healing. These levels, ranging from LOE-1 to LOE-5, are based on methodology and study design. In brief, these were how LOEs were assigned as follows: LOE 1 = randomized control trial; LOE-2 = cohort study; LOE-3 = case-control study; LOE-4 = Case series study; LOE-5 = expert opinion or case report.
The clinical studies were further evaluated for their study design and assessed for the direction of the main conclusion regarding the efficacy of the ES method used. To aid to this process, the following data were extracted from the clinical studies: (1) primary outcome measure, (2) assessment time—time over which ES was monitored, (3) ES treatment regime, (4) main findings, and (5) main conclusion drawn by the authors. A grade of recommendation was then assigned according to Oxford Centre for Evidence Based Medicine guidelines based on the findings for each mode of ES for different clinical situations (Table (Table2).2). In brief, the criteria used was as follows:


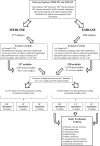
Figure Figure22 shows an overview of the selection of the studies used and the final articles selected for each type of ES for both clinical and in vitro studies.
RESULTS
Mechanism of action of ES
The in vitro studies evaluated report that DC stimulates osteogenesis by an electrochemical reaction at the cathode (O2 + 2H2O + 4e− → 4OH) creating end products referred to as faradic products.13–22 The production of hydroxyl ions (OH) at the cathode are shown to lower the oxygen concentration and increase the pH.15 This environment prevents bone resorption and increase bone formation by increasing osteoblast and decreasing osteoclast action.15 A second faradic product hydrogen peroxide (H2O2) is also formed at the cathode,15 which enhances osteoclast differentiation.20 The resorption by the osteoclasts in turn triggers bone formation by the osteoblasts. The effect of H2O2 could also be due to its stimulatory action on vascular endothelial growth factor secretion by macrophages, which is important for angiogenesis in fracture healing.18 Evidence also shows that DCs’ stimulatory effect may be due to an increase in growth factor synthesis by osteoblasts, in particular bone morphogenetic protein (BMP)-2,6,7.19 Figure Figure3a3a shows a summary of DC-proposed mechanism of action.
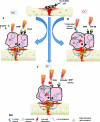
The vitro studies reviewed23–26 that use CC describe the main mechanism by which CC stimulates bone formation is by calcium translocation via voltage-gated calcium channels.23,24 This mechanism was proved when verapamil was administered to block the Ca2+ channels in osteoblasts treated with CC, as the cell proliferation consequently decreased.24 However, once the calcium voltage-gated channels are activated, this triggers an augmenting pathway. First, there is an increase in phospholipase A2, which raises prostaglandin E2 synthesis.23 This then amplifies cystolic Ca2+, which increases intracellular calcium stores to activate the last step in the pathway, of enhancing the activated calmodulin levels.23 Activated calmodulin has been shown to promote cellular proliferation in bone by upregulating nucleotide synthesis and a wide array of enzymatic proteins, which enhances callus formation and maturation.23 Studies also report that CC enhances bone healing by the activation of growth factors, for example, mRNA expression of BMP-2,4,5,6,725 and transforming growth factor-beta 1 (TGF-β1) is increased by osteoblasts stimulated by CC.26 Figure Figure3b3b shows a summary of CC-proposed mechanism of action.
Two mechanisms are described by which IC has its stimulatory effect.23,27–47 First, IC exhibits its effect on bone healing by increasing the calcium uptake of bone. This is achieved by inactivating its signal to parathyroid hormone (PTH)30,31 by preventing the store of cyclic adenosine monophosphate to build up, which is naturally associated with PTH stimulation and the expression of PTH on the cell surface membrane.32 Second, a key metabolic pathway for IC stimulation is the activation of intracellular calcium stores.23 These stores then increase activated calmodulin levels, which enhance osteoblast cell proliferation. This is the key difference to CC, where the activation of intracellular calcium is from an extracellular pathway.23 Thirteen studies35–47 reported that IC stimulates healing by upregulation of growth factor production including BMP-2,4,6,7, TGF-β1, and insulin growth factor-2 by osteoblasts. Figure Figure3c3c shows a summary of IC-proposed mechanism of action.
LOE and efficacy of ES to enhance bone healing
Direct current has been utilized to aid bone healing in spinal fusion, nonunions, delayed unions, and as an adjunct for promotion of bone healing in ankle surgery (Table (Table33)48–81. Four studies supplied LOE-1 for utilizing DC in the treatment of spinal fusion. Direct current was found to be highly effective in the enhancement of failed spinal fusion and as an adjunct to spinal instrumentation.51 However, one study found no difference in fusion success after DC49 and another LOE-1 study showed no increase in lumbar fusion rates in patients older than 60 years after DC.48 Further LOE-252 proved DC to be effectively employed in lumbar interbody fusion. Direct current has been effectively used as an adjunct in hindfoot fusion62 and revision ankle arthrosis nevertheless providing only a LOE-467. The use of DC for nonunion and delayed union is limited again by just LOE-4. LOE-2 supported the use of DC in osteonecrosis of the femoral head.12,53

Capacitive coupling has been used to enhance bone healing in nonunions, delayed unions, and spinal fusion (Table (Table44)82–92. Two LOE-1 studies utilized CC for the treatment of nonunions. The first study84 showed CC to be highly effective for treating long bone nonunion, but the second study used it for tibial stress fractures,82 finding no improvement in healing time. These findings were enhanced by an LOE-4 study where athletes with lower limb stress fractures were successfully treated with CC.86 Furthermore, LOE-4 showed that CC was effective in healing nonunions,87,89,90 whereas LOE-1 has shown CC to enhance lumbar spinal fusion.83

Inductive coupling is extensively utilized for bone healing with 18 LOE-1 studies (Table (Table55)93–146. Three LOE-1 studies utilized IC for tibial nonunions. The earliest study93showed no statistical difference in healing after stimulation, while later studies supported IC.8,108 Furthermore, LOE-4 demonstrated IC to be effective in enhancement of long bone nonunions.123,114,110 LOE-1 studies showed IC to be effective in enhancing healing of femoral9 and tibial osteotomies.104,109 LOE-1 proved IC ineffective for disuse of osteoporosis and bone formation during limb lengthening.97 Inductive coupling has been shown to aid healing of fresh fractures by LOE-1.11,94 Inductive coupling was supported by LOE-1 to be effective for patients undergoing interbody fusion,106 enhancing posterolateral lumbar fusion105 and increasing fusion rates in anterior cervical disectomy.98 LOE-1 and LOE-4 verified that IC is successful in congenital pseudoarthrosis.107 In contrast, LOE-1 proved IC ineffective for Perthes disease.99 Inductive coupling has shown to effectively enhance fusion success of hindfoot arthrodesis with one LOE-1 study96; nonetheless, there is conflicting inconsistent LOE-4 supporting IC for aiding fusion after ankle arthrodesis.137,139,140The results of this study were used to assign grades of recommendations (Table (Table2).2). There was, however, wide study heterogeneity (Table (Table66).


DISCUSSION
Mechanisms of action of ES
The exact mechanism by which ES enhances bone repair remains underexplored. Direct current was shown to work by an electrochemical reaction at the cathode.13–22 For CC, molecular pathways and growth factors have been shown to enhance proliferation and differentiation of the osteoblast.23–26 Inductive coupling was shown to enhance osteoblast differentiation and proliferation by mechanisms involving alteration of growth factors27, gene expression,28 and transmembrane signaling.29 Calcium is upregulated by IC and CC, which is important in bone healing, as it has a role in the mineralization of bone and conducts the communication between cell surface receptors, antibodies, and hormones for DNA synthesis needed for bone healing.33,34The upregulation of growth factor synthesis by all modes of ES acts similarly to enhance bone healing. They work in an autocrine and paracrine action46 to increase the cellular matrix synthesis and gene expression, which in turn increases bone cellular proliferation and differentiation, leading to enhanced callus formation and maturation.36,37 An overview of the mechanisms for ES is shown in Figure Figure3.3. With better understanding of the effect of ES at a molecular level, the effectiveness of ES for enhancement of bone healing in the clinical setting will be improved.
Direct current
Using DC for spinal fusion has shown to be inconsistent with 2 LOE-1 studies51,50 supporting its efficacy particularly in high risk patients (smokers, those with multiple back surgeries, and multilevel fusions) and 2 LOE-1 studies showing no difference in the older patient population leaving DC only level B recommendation.48,49 However, one meta-analysis supports continuous 24-hour delivery of 5 to 10 µA using 2 to 4 cathodes to be effective for spinal fusion.147 Therefore, more studies should be carried out to support DC for spinal fusion. Moreover, DC is effective as an adjunct to foot and ankle surgery with only a level C recommendation. Because of LOE-4 being solely reported, more evidence is required because of a wide range in follow-up (9-20 weeks), small patient population, and large differences in number of surgical inventions before DC was used (range, 1-5). No studies for DC fulfill the criteria for randomized prospective double-blind clinical trial because it would involve implantation of a placebo stimulator, which is against the regulation of human research; therefore, its effect on bone healing remains questionable leaving DC only as a recommendation C for nonunion. LOE-4 supports using DC for the application of enhancing nonunions, and bone healing rates were not affected by the presence of previous osteomyelitis or the presence of previously inserted metallic fixation devices.54 Furthermore, rate of unions were not significantly different compared to rates after bone graft surgery.54 A LOE-4 study showed 10 years after DC stimulation that all fractures had remained united with normal bone remodeling, illustrating that DC is safe and effective in the long term.59However, despite its effectiveness and availability, DC has fallen out of favor compared to IC and CC. Furthermore, IC and CC are noninvasive techniques affected by patient compliance unlike DC.
Capacitive coupling
Using CC for bone healing is limited with only 2 LOE-1 studies. These studies are unreliable, as the success of CC for healing long bone nonunions by Scott and King84 consisted of a small sample size and had a large variety in fracture sites between control and stimulated groups. Despite Beck et al82 reporting good use of randomization, and blinding the outcome assessors, 86% of the patients being followed up showed no difference in the time for healing between the control and CC group. Encouragingly, LOE-4 has demonstrated CC to be effective in treating nonunions,85,87,90 though this unreliable evidence suggests that this application warrants further investigation leaving CC as level of recommendation as C. Using CC for spinal fusion is relatively new, with limited evidence supporting its effectiveness83; therefore, further studies are required though as to date giving a level of recommendation as A.
Inductive coupling
The use of ICs for treating nonunion and delayed union has been successful.8,108 However, in the study by Sharrard,8 the age of the active group was 34.7 and in the control group, it was 45.4. Furthermore, when the results of the study of Simonis et al108 were adjusted for smoking, no enhancing effects were seen for IC. These limitations suggest that further clinical evidence is needed to support this application, as shown by the level of recommendation being C. Inductive coupling has been shown to be beneficial for osteotomies, but the endpoint assessment was shown to vary in 3 randomized controlled trials (RCTs) making true comparisons difficult giving an overall recommendation of B.9,104,109 Inductive coupling is effective in fresh fractures although supported by only 2 RCTs.11,94 In one such study, scintimetric analysis was a primary outcome measure.11 This did not reliably examine the effect of ES on patient’s clinical outcomes and also failed to show any benefit in redisplacement rates between the groups. The subsequent study was limited, as the findings were based on a subgroup of patients using the device for more than 6 hours daily; therefore, the compliance of the patients was influential.94 Hence, overall, the recommendation remains as level B. Inductive coupling had no effect on regenerate bone during limb lengthening,94 even though bone loss in the segments of bone distal to the lengthening sites was significantly more marked using inactive coils, illustrating that IC can prevent bone loss adjacent to the distraction gap. However, multiple limbs were analyzed for the same patient, and the small population decreased the reliability of the results. Two RCTs agreed in supporting IC for enhancing spinal fusion, showing a recommendation of level A.106,107However, in one study, the radiographic criteria for fusion required only 50% incorporation of the graft106and follow-up in both studies was less than a year making definite judgment difficult. Only one LOE-1 study verified IC for congenital pseudoarthrosis,107 which was limited by not blinding the assessor or patients, introducing detection and performance bias, and hence better designed studies are needed giving overall assessment of recommendation of C.
Bone grafting procedures for nonunions is shown to be less successful with multiple graft procedures.145However, bone grafting with ES has shown to yield good results only failing to enhance bone healing in 10% to 15% of cases.145 Bone grafting with DC79 and IC145 has also been shown to be effective in enhancing bone healing. Nonetheless, only 2 studies were found to use this technique.
There were certain limitations found in the studies during evaluation including the following:
-
Randomization of the RCTs was generally maintained but the allocation methods was not well-defined for the RCTs.8,9,11,93,97,107 The dropout rates were adequate being less than 26% on average with follow-up of the patient in the RCTs being nearly all greater than 86%,9,11,84,93,97,108although few studies described the statistical power of their studies.8,84,93 Therefore, more prospective, appropriately powered, well-designed, randomized clinical control trials are needed demonstrating the efficacy of ES in enhancing bone healing in nonunions, delayed unions, fresh fractures, osteotomies, and spinal fusion.
-
Outcome measures for the studies varied, including clinical or radiological union or combinations of both, bone density, scintimetric values, healing success rates, and time to weight bear or consolidation (Table (Table6).6). Very few studies looked at patient outcomes, including pain, need for revision surgery, and improvement in functional status. The few studies that addressed the effect of ES on patient outcome showed no benefit in terms of pain.8,56,61
-
Furthermore, there is lack of agreement of a definition for a nonunion varying from 3 months to 9 months and the entry criteria amongst studies (see Tables 3–5).84,85,88,91Some studies define nonunion clinically, whereas some additionally incorporate radiological criteria.84,85,91 Therefore, a consensus is needed for the definition of nonunion, and thus ES studies can be reliably compared.
-
The length of assessment to assess the effectiveness of ES also varied from 2 to 18 months (Table (Table6).6). The treatment time also varied as shown in Table Table6.6. Capacitive coupling treatment ranged from 10 weeks to 6 months for between 10 and 24 hours a day and IC ranged from 3 to 18 hours daily over a period of 3 weeks to 9 months with DC being more uniform at 12 weeks.
-
There was a degree of variety in frequency and amplitude within the type of ES subgroups (Tables 3–5). Most IC devices reported a similar frequency between 15 and 75 kHz.9,10,11,93,94,110,114,115,119 Direct current was generally reported using 20 µA across 4 cathodes53,54,55,56 though there was heterogeneity, as 2 studies reported 40 µA48,65 and 3 studies used 10 µA.51,57,75 Capacitive coupling in approximately half of the studies reported at 60 kHz at 5 V.82,87,88,90,91
-
Few trials had more than 80 patients; for example, there were only 10 patients in DC and IC and 1 patient in CC, which was for spinal fusion (see patient number in Tables 3–5).
In conclusion, the exact mechanism by which ES enhances bone repair is still not fully understood and needs more investigation. However, to date, DC has been documented to work by an electrochemical reaction at the cathode, and CC and IC have shown to work by alteration of growth factors and transmembrane signaling. In an era of evidence-based medicine, fracture-healing management should be based on the best available evidence to ensure high-quality, safe, and cost-effective treatment. Therefore, considering the widespread usage of ES to aid bone healing in clinical practice, our analysis shows that there have been few good quality clinical studies to support their use. This is demonstrated by the low recommendation assigned to ES for different orthopedic conditions requiring bone healing except spinal fusion (Table (Table2).2). Therefore, the optimal regime for ES treatment for bone healing needs to be further defined and standardized. Moreover, clinical studies need to have uniform outcomes and defined criteria on the effect of ES on clinical outcomes including improvement in pain, activities of daily living, and need for revision surgery. Overall, the evidence to date implies that further studies are needed to support and optimize the clinical application of ES for bone healing.
REFERENCES
