Significance: Natural, endogenous electric fields (EFs) and currents arise spontaneously after wounding of many tissues, especially epithelia, and are necessary for normal healing. This wound electrical activity is a long-lasting and regulated response. Enhancing or inhibiting this electrical activity increases or decreases wound healing, respectively. Cells that are responsible for wound closure such as corneal epithelial cells or skin keratinocytes migrate directionally in EFs of physiological magnitude. However, the mechanisms of how the wound electrical response is initiated and regulated remain unclear.
Recent Advances: Wound EFs and currents appear to arise by ion channel up-regulation and redistribution, which are perhaps triggered by an intracellular calcium wave or cell depolarization. We discuss the possibility of stimulation of wound healing via pharmacological enhancement of the wound electric signal by stimulation of ion pumping.
Critical Issues: Chronic wounds are a major problem in the elderly and diabetic patient. Any strategy to stimulate wound healing in these patients is desirable. Applying electrical stimulation directly is problematic, but pharmacological enhancement of the wound signal may be a promising strategy.
Future Directions: Understanding the molecular regulation of wound electric signals may reveal some fundamental mechanisms in wound healing. Manipulating fluxes of ions and electric currents at wounds might offer new approaches to achieve better wound healing and to heal chronic wounds.

Scope and Significance
Many tissues in their normal healthy state generate electrical potentials by directional transport of ions. Injury to these tissues produces significant electric fields (EFs) and currents that have been proposed to play roles in subsequent healing by stimulating division, proliferation, and directional migration of cells into the wound. Here, we review the current knowledge of the origins of the wound electrical response, including roles of ion channels, cell depolarization, gap junctions, and calcium signaling. We focus on the cornea as a wound-healing model, although these fundamental mechanisms of wounds and wound healing such as EFs and currents, ion channels and ion flux, cell division, proliferation, and migration are applicable to many wounds, including skin.
Translational Relevance
Endogenous wound electric signals were discovered many years ago. Using modern techniques, the existence of these natural EFs and currents have been confirmed. It has been suggested that the natural electric signals might have a role in wound healing. Endogenous EFs have also been implicated in development and regeneration. Both skin keratinocytes and corneal epithelial cells respond robustly to EFs of a strength that are measured at wounds. Most importantly, electric signals of physiological strength are a predominant guidance cue directing cell migration in wound healing, over-riding other well-accepted directional cues.
Clinical Relevance
Delayed or non-healing wounds pose an immense health and economic problem, with an estimated cost to the U.S. health system in excess of $20 billion. Effective wound healing requires well-controlled cell movement and tissue growth. However, the basic mechanisms of epithelial repair are not fully understood. Understanding the molecular mechanisms of the initiation and regulation of the wound electrical response, and its role in wound healing, will open a new avenue to treat delayed and nonhealing skin and corneal wounds, and wounds in general.
Introduction
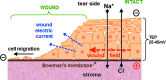
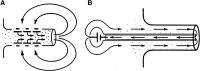
Most cells are electrically active in that they generate a membrane potential. This electrical potential, which is negative inside the cell compared with the outside, is generated by active pumping of ions across the membrane. The membrane potential enables cells to funcion as a battery, providing power to various proteins in the membrane, for example, to generate adenosine triphosphate. Excitable cells such as neurons have very large membrane potentials that they use for transmitting signals in the form of a cascading depolarization called the action potential. Many tissues and organs in their normal state are also electrically active. For example, cornea, skin, ocular lens, and many others generate significant electrical potentials by selective and directional pumping of ions.1–7 These potentials are necessary for normal function, for example, to maintain water balance and transparency in the cornea, and to generate a micro-circulatory system in the avascular lens. These epithelial tissues, analogous to the cell membrane, also act as a high resistance barrier to maintain the trans-epithelial potential (TEP) by minimizing passive flow of ions down their concentration gradients. For example, epithelial cells in the cornea and skin are connected by tight junctions.8,9 Injury to the cornea disrupts this high resistance barrier, and the TEP collapses to zero at the wound site (Fig. 1).10 The TEP in the surrounding intact epithelium acts similar to a battery to generate significant EFs around (and orientated into) the site of injury (Fig. 1; red arrow), and also generates large electric currents flowing out of the wound (Fig. 1; blue arrows). After corneal injury, the wound EF increases and is maintained for many hours in what appears to be a regulated and controlled wound response. The cornea wound EFs can be increased or decreased by modulating ion transport, which, in turn, enhances or inhibits wound healing.11 These EFs have, therefore, been implicated in stimulation of cell migration, division, and proliferation to promote wound healing.11–16
 mV, which …
mV, which …The responses of plant and animal cells to applied EFs were first studied in the late nineteenth and early twentieth centuries (for a review of early work, see Jaffe and Nuccitelli17). Later studies refined the technique to use a more physiological EF and minimize artefacts such as electrode products, flow problems, and temperature changes. Neurites, neural crest cells, fibroblasts, epithelial cells, and other cell types migrated to the cathode.18–22 Some cells did not respond or migrated to the anode.23 Theories on the mechanism of how cells detect these physiological EFs include actin alignment,24 re-distribution of proteins (receptors, ion channels, etc.) in the cell membrane,25,26 and organelle polarization.27,28Intracellular signaling pathways are also involved.16,29–31 See these reviews for more detailed information.15,32–37 The search for the molecular and genetic “electric sensing” mechanism continues.
Although wound EFs and currents were first discovered and measured many years ago, the molecular mechanisms of how they are initiated and regulated to stimulate wound healing remain unclear. In this review, we bring together the current understanding of various factors that are involved in the generation of wound electrical activity, including ion channels, gap junctions, membrane potential, and calcium signaling.
Wound Electric Signal and Ion Flux
Cornea as a wound-healing model
Many studies on wounds and wound healing have used the cornea as a model system.11,14,16,29,31,37–43Cornea wounds not only have clinical relevance (e.g., in diabetes and dry eye), but also, at the cellular and tissue level, many factors involved in the wound response and wound healing are common to other tissues, including skin wounds. These include wound void, cell damage/depolarization, calcium signaling, EFs, ion channels and pumps, ion fluxes, chemotaxis, cell division, and cell migration. Wounds of the corneal epithelium heal by proliferation and migration of epithelial cells adjacent to the wound.44 Wing layer cells at the wound edge flatten and slide, leading to covering of the abraded area with a thin layer of epithelial cells in 1 to 4 days. Epithelial cells around the wound begin to replicate within 24 h after injury and migrate into the wound, restoring the epithelial layer to its normal thickness.
h after injury and migrate into the wound, restoring the epithelial layer to its normal thickness.
The cornea has been valuable as an injury and wound healing model, because (1) ex vivo eyes are readily accessible for electrophysiology and for wound healing assays; (2) the cornea can be readily exposed to different ionic regimes or pharmacological drugs (this can also be done by eye drops in living animals in vivo);11,45 (3) the cornea wound depth is precicely controlled by removing the epithelium down to the basement (Bowman’s) membrane, and different sizes or shapes of wounds can be made; (4) wound-healing assays can be done with ex vivo eyes in organ culture or in whole living animals in vivo using fluorescein for wound imaging;11,31 and (5) corneal epithelial cells can be readily isolated and cultured for application of EFs in electrotaxis experiments or for patch clamp electrophysiology.46,47 It is also possible, though technically challenging, to do electrophysiology (e.g., measure wound current or ion flux) in corneas of living, anesthetized animals (Reid and Zhao, unpublished data, University of California, Davis, 2010).
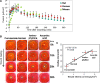
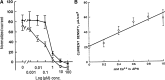
Measuring cornea wound electric current in an artificial tear solution similar to normal tears gives very consistent results with a rapidly rising outward current that is maintained for many hours (Fig. 2A).11,45These currents measured in bulk solution are probably less than would be seen in vivo not only due to the temperature difference (Room temperature vs. ∼30°C) but also because usually these currents would flow through the narrow tear film, thus increasing the current density. Skin wound currents also produce large outward currents, but almost always with an initial transient inward current; for example, see Figure 4 in Reid et al., 2007.48 However, measuring skin wounds in saline solution is somewhat artificial; usually, the skin is dry with the outward wound current flowing not at the surface as in the cornea but through the epidermis between the stratum corneum and stratum granulosum.12 Skin wounds are also less amenable to ion and drug manipulations. Corneal epithelial cells migrate in applied EFs of physiological magnitude46,47 and actually respond better in coordinated groups than as individuals.49 Skin wound healing is more complex, involving more cell types than the cornea (see section Chronic skin wounds and electrical stimulation below for a detailed description of skin wound healing). However, isolated skin cells (e.g., keratinocytes) are very sensitive to applied EFs, responding rapidly to very small physiological EFs.50

 μm. (B)Chloride currents recorded from primary cultured HCE cells. Left:whole-cell chloride current at different …
μm. (B)Chloride currents recorded from primary cultured HCE cells. Left:whole-cell chloride current at different …Regulated wound currents in cornea
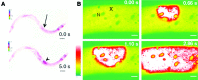
The vibrating probe48,51 has been an extremely useful tool to measure non-invasively wound electric currents in a variety of systems and tissues, including skin, cornea, and ocular lens.6,11,48 The electrical response of the cornea wound is consistent and repeatable, and is comparable in mouse, rat, and human corneas (Fig. 2A).11,45 The intact, unwounded cornea current is very small (shown at time zero in Fig. 2A). On wounding by scraping away a small area of epithelium down to the basement membrane, there is a rapid rise of electric current at the wound edge, peaking at almost 10 μA/cm2 40
μA/cm2 40 min after wounding in rat cornea. This is followed by a slow decline, but the current stays above the unwounded level for at least 6
min after wounding in rat cornea. This is followed by a slow decline, but the current stays above the unwounded level for at least 6 h after wounding. This pattern of electrical activity after wounding is similar in human post mortem cornea, and in mouse cornea, although the currents are smaller. EFs of approximately 42
h after wounding. This pattern of electrical activity after wounding is similar in human post mortem cornea, and in mouse cornea, although the currents are smaller. EFs of approximately 42 mV/mm have been measured at the bovine cornea wound edge by direct measurement with electrodes soon after wounding.52 These large, long-lasting electric currents and fields at the cornea wound appear to be an active response, and are necessary for normal wound healing. Pharmacological drugs that inhibit (furosemide) or enhance (aminophylline, ascorbic acid) ion transport can decrease or increase wound current and wound healing, respectively (Fig. 2B).11,13There is a strong correlation between the magnitude of the endogenous wound electric current and the rate of wound healing (linear best fit value R2=0.95) (Fig. 2C). In addition, corneal epithelial cells migrate to the cathode in applied EFs of physiological magnitude.46,47 These data strongly suggest that cells use the wound EFs and currents as a directional cue to migrate directionally into the wound to initiate and promote healing.
mV/mm have been measured at the bovine cornea wound edge by direct measurement with electrodes soon after wounding.52 These large, long-lasting electric currents and fields at the cornea wound appear to be an active response, and are necessary for normal wound healing. Pharmacological drugs that inhibit (furosemide) or enhance (aminophylline, ascorbic acid) ion transport can decrease or increase wound current and wound healing, respectively (Fig. 2B).11,13There is a strong correlation between the magnitude of the endogenous wound electric current and the rate of wound healing (linear best fit value R2=0.95) (Fig. 2C). In addition, corneal epithelial cells migrate to the cathode in applied EFs of physiological magnitude.46,47 These data strongly suggest that cells use the wound EFs and currents as a directional cue to migrate directionally into the wound to initiate and promote healing.
Ion flux in cornea and skin
Wound EFs and currents are produced by directional flow (flux) of charged ion species that are present in cell cytoplasm and intercellular fluid (Na+, Cl−, K+, Ca2+ and others). Some of this ion flux will be leakage of ions from damaged cells and tissues soon after injury, but large currents can be measured for many hours and days after wounding, suggesting that there may be regulation of ion transport as a part of the wound response. To help understand this wound electric response, Vieira et al. characterized these ion fluxes in rat cornea using ion-selective microelectrodes.10 The largest ion flux observed at rat cornea wounds was an inward chloride flux (influx), with a smaller component of inward sodium flux (Fig. 3A).10 Enhancement of wound electric current by aminophylline (see Fig. 2C) was partly by reversal of sodium flux (to outward efflux), but mainly by enhancement of the inward chloride flux. Adding together all the ion fluxes (Cl−, Na+, Ca2+, and K+) from timelapse measurements showed that increasing total ion flux correlated with the increase of electric current after wounding (Fig. 3B). Reid and Zhao measured ion flux and electric current at human skin wounds in vivo (unpublished observations University of California, Davis, 2008, 2011). They saw a very large efflux of sodium and influx of chloride, with smaller fluxes of calcium and hydrogen. Interestingly, the human skin wound electric current remained above unwounded levels for 4 days, indicating that it is present not just soon after wounding, but also during the early phases of skin wound healing.
Chronic Skin Wounds and Electrical Stimulation
Skin wound healing is a dynamic and complex process involving soluble factors, extracellular matrix, blood cells, and various skin cells (e.g., keratinocytes, fibroblasts, macrophages, endothelial cells).53 There are three overlapping phases: inflammation, tissue formation, and tissue remodeling. The first phase covers the wound with a blood clot. Platelets release platelet growth factor, which activates macrophages and fibroblasts. Neutrophils clean the wound of cell debris. Adherence to the extracellular matrix stimulates cells to undergo metamorphosis and to release additional growth factors, chemoattractants, and cytokines. Re-epithelialization begins within hours, and cells undergo phenotypic changes that facilitate migration. Expression of integrin receptors on epidermal cells allows them to interact with a variety of extracellular-matrix proteins such as fibronectin. Epidermal cells migrate between the dead tissue (blood clot above) and healthy tissue below. One or two days later, epidermal cells at the wound edge behind the migrating cells begin to divide and proliferate. New stroma (granulation tissue containing new capillaries) begins entering the wound at about 4 days. Fibroblasts continue to deposit and remodel the extracellular matrix, and fibroblast growth factor (and other growth factors) stimulates new blood vessel formation. Wound contraction occurs in the second week, and the wound gains strength by accumulation and remodeling of collagen.
The detection many years ago of significant skin wound electric potentials led to the hypothesis that the skin wound EF was involved in the healing process.12,16,34,36,54,55 Intact healthy skin generates and maintains a significant electrical TEP (up to 100 mV),4 and skin wounds quickly generate large and persistent wound electric currents and fields.3,4,12,48Skin keratinocytes and fibroblasts migrate in physiological EFs50,56,57 as do some immune cells, for example, lymphocytes.58 Other more specialized skin cells also respond to applied EFs, for example, vascular endothelial cells59,60 and microvascular cells,23 which are responsible for new blood vessel formation. Neurons also grow in response to skin wounds,61 and nerve growth toward cornea wounds is stimulated and directed by the endogenous wound electric signal.62 These results led to the hypothesis that applied EFs may help heal chronic nonhealing wounds where other treatments have failed.54
mV),4 and skin wounds quickly generate large and persistent wound electric currents and fields.3,4,12,48Skin keratinocytes and fibroblasts migrate in physiological EFs50,56,57 as do some immune cells, for example, lymphocytes.58 Other more specialized skin cells also respond to applied EFs, for example, vascular endothelial cells59,60 and microvascular cells,23 which are responsible for new blood vessel formation. Neurons also grow in response to skin wounds,61 and nerve growth toward cornea wounds is stimulated and directed by the endogenous wound electric signal.62 These results led to the hypothesis that applied EFs may help heal chronic nonhealing wounds where other treatments have failed.54
Chronic wounds are long-lasting wounds that heal very slowly or do not heal at all. The most common of the chronic wounds are pressure ulcers (decubitus ulcers, commonly known as bedsores). The goal of treating skin ulcers is twofold: correction of the cause and stimulation of healing.63 Lack of effective treatment options and accumulating evidence suggesting that the natural endogenous skin wound EF stimulates wound healing has led to a number of different studies investigating the effect of electrical stimulation on chronic wound healing. Several different methodologies have been used for electrical stimulation in chronic wound healing. For example: (1) direct current (DC), continuous stimulation for 1 s or longer; (2) pulsed current (PC), brief unidirectional or bidirectional stimulation in which each pulse is separated by a longer off period of no current flow, includes low-frequency PC and high-voltage PC (HVPC); (3) alternating current (AC), electric current in which the direction of the flow is reversed at frequent intervals. There are also differences in voltage, time interval between treatments, length of treatments, and different number of treatments. It is beyond the scope of this review to summarize all the literature on electrical stimulation of chronic skin wounds. However, a number of studies on chronic skin wounds have shown significant improvements of healing by (1) high-voltage (100–175 V) monophasic pulsed (105
s or longer; (2) pulsed current (PC), brief unidirectional or bidirectional stimulation in which each pulse is separated by a longer off period of no current flow, includes low-frequency PC and high-voltage PC (HVPC); (3) alternating current (AC), electric current in which the direction of the flow is reversed at frequent intervals. There are also differences in voltage, time interval between treatments, length of treatments, and different number of treatments. It is beyond the scope of this review to summarize all the literature on electrical stimulation of chronic skin wounds. However, a number of studies on chronic skin wounds have shown significant improvements of healing by (1) high-voltage (100–175 V) monophasic pulsed (105 Hz) current for 45
Hz) current for 45 min per day,64 (2) pulsed (0.8
min per day,64 (2) pulsed (0.8 Hz) low-intensity (300–600
Hz) low-intensity (300–600 μA) DC three times per day for 3 days per week,65 (3) pulsed voltage (50 V at 80
μA) DC three times per day for 3 days per week,65 (3) pulsed voltage (50 V at 80 Hz for 10
Hz for 10 min followed by 50 V at 8
min followed by 50 V at 8 Hz for 10
Hz for 10 min, repeated for 8
min, repeated for 8 h per day),66 (4) HVPC (150 V, 100
h per day),66 (4) HVPC (150 V, 100 Hz, 100
Hz, 100 μs) for 45
μs) for 45 min, three times a week, for 4 weeks,67 (5) AC (7–10 mA, 40
min, three times a week, for 4 weeks,67 (5) AC (7–10 mA, 40 Hz) or DC (0.6 mA) stimulation,68 and pulsed electromagnetic field (bidirectional pulses of 3.5
Hz) or DC (0.6 mA) stimulation,68 and pulsed electromagnetic field (bidirectional pulses of 3.5 ms at 0.06
ms at 0.06 mV/cm.69 See also additional reviews and literature surveys.70–75 The voltages applied are higher than the endogenous human skin wound EF, which is in the range of 100–300
mV/cm.69 See also additional reviews and literature surveys.70–75 The voltages applied are higher than the endogenous human skin wound EF, which is in the range of 100–300 mV/mm.76 This is because the high resistance of the skin means there will be a significant drop of voltage between the electrodes and the cells migrating at the wound edge to heal the wound.
mV/mm.76 This is because the high resistance of the skin means there will be a significant drop of voltage between the electrodes and the cells migrating at the wound edge to heal the wound.
Problems of EF application to treat chronic skin wounds include (1) Heat production: application of DC voltage (>1 s) to the high-resistance skin generates electrothermal heat that is proportional to the resistance, the square of the current, and the time that the current is applied (Joule’s law: P=I2×R). Thus, the voltage and time should be minimized to avoid electrothermal damage. (2) Chemical production: application of DC voltage can cause chemical reactions at the electrodes, resulting in pH changes, which can cause cell and tissue damage. Again, minimizing the voltage and time of application, or reversing the polarity at regular intervals by using AC, helps minimize these artefacts. Other adverse effects include patient reports of skin tingling and irritation under the electrodes.54 Alternatively, recent technology may allow the electrodes to be eliminated all together by using “wireless” electrical stimulation.77 Application of exogenoue EFs to the cornea is more difficult. Contact lenses with integrated electric circuits have been proposed.78,79Pharmacological stumulation of ion pumping to enhance the transcorneal potential and the wound EF and to enhance wound healing appears to be a promising strategy in the cornea.11,13,16,62 See also the Diabetic disease model section.
s) to the high-resistance skin generates electrothermal heat that is proportional to the resistance, the square of the current, and the time that the current is applied (Joule’s law: P=I2×R). Thus, the voltage and time should be minimized to avoid electrothermal damage. (2) Chemical production: application of DC voltage can cause chemical reactions at the electrodes, resulting in pH changes, which can cause cell and tissue damage. Again, minimizing the voltage and time of application, or reversing the polarity at regular intervals by using AC, helps minimize these artefacts. Other adverse effects include patient reports of skin tingling and irritation under the electrodes.54 Alternatively, recent technology may allow the electrodes to be eliminated all together by using “wireless” electrical stimulation.77 Application of exogenoue EFs to the cornea is more difficult. Contact lenses with integrated electric circuits have been proposed.78,79Pharmacological stumulation of ion pumping to enhance the transcorneal potential and the wound EF and to enhance wound healing appears to be a promising strategy in the cornea.11,13,16,62 See also the Diabetic disease model section.
Regulation of Ion Channels and Pumps at Wounds
Chloride channels in cornea wound healing
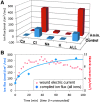
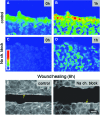
The cornea contains many ion channels and pumps that are involved in homeostatic functions such as transport of nutrients or waste products, and water balance to prevent swelling and maintain transparency.80These channels and pumps generate and maintain the TEP in the normal healthy cornea, but do they have a role in the wound electrical response? Cao et al. studied the distribution and expression of chloride channels (CLCs) in human corneal epithelium (HCE).81 Multiple CLC types were present but showed different patterns of distribution. For example, CLC-3 and 4 were expressed only in apical epithelial cells, CLC-2 in apical and basal cells, and CLC-6 mainly in apical cells but to a lesser level throughout the epithelium, suggesting they have different roles (Fig. 4A). The polarized distribution of these different CLCs also suggests a structural basis of directional chloride transport in the corneal epithelium. Patch clamp recording from HCE cells confirmed the presence of a robust, Ca2+-dependent, Cl− current (Fig. 4B).81 Vieira et al.showed that wounding an HCE cell monolayer causes up-regulation of CLC-2 mRNA (Fig. 4C).10 They also showed that injury to rat cornea caused re-distribution and increased concentration of CLC-2 in the epithelium near the wound (Fig. 4D). Therefore, corneal injury, by some unknown (possibly calcium-dependent) signaling mechanism, triggers CLC up-regulation and activity, increasing Cl− flux and generating the wound electric current.
Wounding activates cation channels
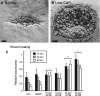
Ion channels in cell membranes are involved in cellular mitogenesis and proliferation events such as cell activation, migration, and responses to growth factors. During wound healing in the rabbit cornea, endothelial cells show many of these channel-mediated events. After wounding, corneal endothelial cells change their morphology and migrate and divide to cover the basement membrane. Ion channels also appear to be involved in the generation of electrical fields around wounds. In rabbit cornea, it was found that a nonselective cation channel is expressed over a 2- to 3-day time period after wounding.82 Corneal endothelial cells from control or sham-wounded corneas had small whole-cell currents, while endothelial cells from wounded corneas had much larger currents (Fig. 5). Pharmacological treatments and ion replacement showed that these currents were produced by nonselective cation channels (carrying mainly Na+and K+). The time scale of the channel activation (2–3 days after wounding) suggests it may be wound-healing specific and related to increased cell migration and responsiveness to wound-related cues (growth factors, EFs).

 h …
h …Sodium and CLCs in Xenopus wound healing
Wound healing in amphibian early embryos involves changes in cell shapes similar to those seen in normal morphogenesis, and it is initiated by a local influx of calcium into cells. Xenopus frog embryo wound healing appears to involve coordinated changes in cell shape, probably via activation of microfilaments.83 Changes in the shapes of cells at the wound margin are correlated with the degree of wound healing, and it is probable that calcium acts as the immediate trigger for these changes in local cell contractility. Shortly (15 min) after wounding a Xenopus embryo (neurula stage 15–16), cells at the wound margin are elongated and tapered toward the wound, and the wound is partially closed (Fig. 6A). In ethylenediaminetetraacetic acid (EDTA) to remove external calcium, wound closure is significantly inhibited (Fig. 6B). Local application of calcium ionophore A23187 (which makes cell membranes freely permeable to Ca2+) to unwounded embryos also invoked cell shape changes, mimicking the presence of a wound. Fuchigami et al. showed that low sodium and/or chloride in the bathing solution accelerated wound healing in Xenopus embryos.84 They went on to investigate the roles of chloride and sodium (and their respective channels) in wound healing.85 Specific blockage of epithelial sodium channel (ENaC) or CLC-3 reduced the rate of wound closure. The co-presence of both inhibitors of EnaC and CLC-3 (amiloride and 5-nitro-1-(3-phenylpropylamino) benzoic acid respectively) caused a remarkable reduction in the rate of wound closure (Fig. 6C). Lanthanam chloride, which blocks Ca2+ channels (but also the K+ and Cl− channel), also significantly inhibited Xenopusembryonic wound closure. This suggests the participation of multiple ion channel/transporter types in amphibian wound healing. There was a local enhancement of CLC-3 expression at the leading edge of the wounded epidermis in Xenopus embryos as the wound was healing (30–60
min) after wounding a Xenopus embryo (neurula stage 15–16), cells at the wound margin are elongated and tapered toward the wound, and the wound is partially closed (Fig. 6A). In ethylenediaminetetraacetic acid (EDTA) to remove external calcium, wound closure is significantly inhibited (Fig. 6B). Local application of calcium ionophore A23187 (which makes cell membranes freely permeable to Ca2+) to unwounded embryos also invoked cell shape changes, mimicking the presence of a wound. Fuchigami et al. showed that low sodium and/or chloride in the bathing solution accelerated wound healing in Xenopus embryos.84 They went on to investigate the roles of chloride and sodium (and their respective channels) in wound healing.85 Specific blockage of epithelial sodium channel (ENaC) or CLC-3 reduced the rate of wound closure. The co-presence of both inhibitors of EnaC and CLC-3 (amiloride and 5-nitro-1-(3-phenylpropylamino) benzoic acid respectively) caused a remarkable reduction in the rate of wound closure (Fig. 6C). Lanthanam chloride, which blocks Ca2+ channels (but also the K+ and Cl− channel), also significantly inhibited Xenopusembryonic wound closure. This suggests the participation of multiple ion channel/transporter types in amphibian wound healing. There was a local enhancement of CLC-3 expression at the leading edge of the wounded epidermis in Xenopus embryos as the wound was healing (30–60 min after injury). Reid and Zhao measured large (up to 6
min after injury). Reid and Zhao measured large (up to 6 μA/cm2) inward currents at small wounds on Xenopus oocytes (unpublished observations, University of California, Davis, 2012), which may be generated partly by CLCs that are up-regulated at the wound edge.
μA/cm2) inward currents at small wounds on Xenopus oocytes (unpublished observations, University of California, Davis, 2012), which may be generated partly by CLCs that are up-regulated at the wound edge.
 min after wounding in normal solution, cells around the wound edge change shape by elongating …
min after wounding in normal solution, cells around the wound edge change shape by elongating …Newt limb stump currents—sodium channels and calcium regulation
Large, skin-driven electric currents have been measured leaving newt limb stumps during the first 5 to 10 days after amputation.86 These outward currents are balanced by smaller inward currents that enter the intact skin around the stump (Fig. 7A). These currents are driven by (and are dependent on) the skin “electric battery,” similar to the cornea wound EF, which depends on the TEP.11,13 Low sodium, or sodium channel blocker amiloride, in the external solution reduced the size of the limb stump currents. It was also demonstrated by the same authors that frog limb regeneration could be initiated by driving a small steady current through the stump.87 The artificial current was driven by a mercury cell battery implanted in the dorsal lymph sac. Current was pulled through the tissue to the mercury cell from the end of the stump through a Ringer’s solution-soaked wick (Fig. 7B). Implantation of sham stimulators (delivering no current) yielded limbs showing no regeneration but just a healing response. These results suggest that the endogenous stump currents play some causal role in initiating regeneration. Eltinge et al. confirmed that newt limb stump electric currents were sodium dependent and inhibited by sodium channel blocker amiloride (Fig. 8A).88They also showed that the stump currents were dependent on external [Ca2+] (Fig. 8B).
Changes in Membrane Potential, Calcium Signaling, and Gap Junctions
Cell depolarization at wounds
As mentioned earlier (see section Introduction), almost all cells generate and maintain a significant electrical membrane potential that is important for many cell functions. Relatively large and long-lasting changes in membrane potential (depolarization, hyperpolarization) are also important signals in many situations, including the early wound response and wound healing. For example, bovine corneal endothelial cell monolayers heal by a mechanism that combines actin cable formation and cell migration. Depolarization of cells occurs at the leading edge of the wounds and gradually extends inward to neighboring cells.42 This spontaneous depolarization is involved in the development of the characteristic actin reorganization demonstrated by the healing cells. Cells at the wound edge are depolarized in a sodium-dependent way, and the depolarization is passed to neighboring cells away from the wound edge (Fig. 9A, B). Blocking sodium channel ENaC (or replacing external sodium with choline) decreases depolarization (Fig. 9C, D) and actin cable formation, and slows wound healing (Fig. 9E, F). However, replacement of Na+ with Li+ showed that the actin reorganization was not sodium dependent but is triggered electrically by the membrane depolarization. The authors conclude that spontaneous depolarization of the cells at the wound border, regulated by a rise in ENaC activity, constitutes an additional factor in the intermediate cellular processes, leading to wound healing in some epithelia.
Wound calcium signal
Wounding a corneal epithelial cell monolayer activates immediate waves of intracellular calcium signal (Fig. 10A).89,90 Cells at the wound edge show a rapid rise of internal calcium [Ca2+]i which propagates to neighboring cells outward from the wound edge. The calcium wave has two phases: initiation and propagation, and these phases can be decoupled. Thus, removing external calcium prevented initial rise of [Ca2+]i in cells adjacent to wounds but did not prevent propagation of a less intense wave. Thapsigargin, which depletes intracellular calcium stores, did not prevent rise of [Ca2+]i in cells at the wound edge, but inhibited wave propagation. Both phases of the calcium wave response were required for wound healing. Depletion of intracellular calcium stores with thapsigargin or incubation in calcium-free medium almost completely inhibited wound healing (Fig. 10B).
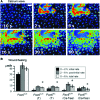
 μm). (B) Depletion …
μm). (B) Depletion …Wounding the skin of the nematode worm Caenorhabditis elegans causes a rapid and maintained rise in epidermal calcium that is essential for survival after wounding (Fig. 11).91The wound-induced rise in calcium requires the epidermal transient receptor potential channel, melastatin family channel gene trap locus 2, and inositol trisphosphate receptor-stimulated release from internal stores. The resultant calcium-dependent signaling cascade promotes wound closure by promoting actin polymerisation at the wound site. Wound closure requires cell division control protein 42 homolog small guanosine triphosphatase and actin-related protein complex-dependent actin polymerization and is negatively regulated by Rho and nonmuscle myosin.
 μm. (B) Femptosecond …
μm. (B) Femptosecond …Gap junctions in cornea healing
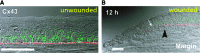
Gap junctions are specialized intercellular connections that are found between many types of animal cells, including corneal epithelial cells. These specialized protein channels directly connect the cytoplasm of two cells, enabling various molecules and ions to pass freely between cells. Gap junctions also allow for direct electrical communication between cells. Gap junction 43 (Cx43) is generally expressed in the basal layers of the rat corneal epithelium, where it regulates proliferation and differentiation. Suzuki et al.92 showed that actively migrating rat cornea epithelial cells lacked Cx43 gap junctions and desmosomes that are usually present in the basal cell layer (Fig. 12). At a rat cornea wound, Cx43 was down-regulated in basal epithelial cells at the leading edge of the migrating epithelium but remained largely unaffected in those of the more remote, noninjured region.93Interestingly, further transient down-regulation of Cx43 using Cx43-specific antisense oligodeoxynucleotides significantly increased the cornea wound-healing rate.94 In addition, Reid and Zhao saw that blocking gap junctions with apigenin significantly increased rat cornea wound electric current (unpublished observations, University of California, Davis, 2011). Down-regulation of gap junctions and desmosomes may, therefore, be a corneal wound response mechanism that facilitates migration of epithelial cells into the wound to initiate and promote healing. The larger wound electric current may be caused by the loss of electrical communication between the cells.
Bone Electric Currents and Healing
Healthy living bone drives an electric current through itself.95 Generation of this endogenous electric current appears to depend to some extent on mechanical stress; mechanical deformation induces electrical stimuli, which subsequently control osteogenic growth.96 In vivo recordings from human tibia while walking have indicated a piezoelectric response as high as 300 mV.97 Bone fracture produces a large but short-lived current and also a smaller prolonged and stable current driven by a cellular battery.95 This bone fracture current is carried mainly by chloride ions; immersion in Cl− free solution reverses the current polarity.95 A hypothetical “pump-leak” model was proposed in which ions (mainly Cl− but also Na+, Ca2+, and Mg2+) are actively pumped into the bone, perhaps across the endosteum. Current at the fracture site is produced by ion leakage, which is maintained by ion pumping in the intact bone.95 Clinical application of currents of the same polarity and similar magnitude to the endogenous bone fracture currents have been successful in treating chronic nonunions in fractured bones.98 Bone marrow cells involved in bone regeneration and healing respond to physiological DC EFs by directional migration.99 Thus, the structural integrity of bone is maintained by anatomical forces that are electrical in nature.
mV.97 Bone fracture produces a large but short-lived current and also a smaller prolonged and stable current driven by a cellular battery.95 This bone fracture current is carried mainly by chloride ions; immersion in Cl− free solution reverses the current polarity.95 A hypothetical “pump-leak” model was proposed in which ions (mainly Cl− but also Na+, Ca2+, and Mg2+) are actively pumped into the bone, perhaps across the endosteum. Current at the fracture site is produced by ion leakage, which is maintained by ion pumping in the intact bone.95 Clinical application of currents of the same polarity and similar magnitude to the endogenous bone fracture currents have been successful in treating chronic nonunions in fractured bones.98 Bone marrow cells involved in bone regeneration and healing respond to physiological DC EFs by directional migration.99 Thus, the structural integrity of bone is maintained by anatomical forces that are electrical in nature.
Diabetic Disease Model
Diabetic patients have poor circulation, often neuropathy (damaged nerves), and a weakened immune system. These factors make them susceptible to chronic slow- or non-healing injuries, particularly in the skin of the lower limbs.100,101 Wound healing is also impaired in diabetic cornea.102 The diabetic wound healing defect may be partly due to factors such as altered growth factor activation and cellular activity. Microcirculatory changes, neuropathy, and altered extracellular matrix and cytokine production have also been implicated.103 There appears to be little published work on the electrophysiology of diabetes, apart from measurements of abnormal nerve function due to diabetic neuropathy.104Ionescu-Tírgovişte et al.105measured skin electrical potential between two acupuncture needles in the skin. They showed a lower mean value of electrical potentials in diabetic patients with clinical signs of neuropathy compared with those without clinical signs of neuropathy. This may suggest that diabetic skin has a lower electrical TEP and therefore smaller wound currents, which, in turn, would explain the reduced wound healing in diabetic patients.
There have been a number of reports of electrical stimulation accelerating wound healing in diabetic patients with chronic nonhealing wounds.54,75 Application of high-voltage pulsed currents once or twice per day, combined with standard wound care, significantly improved healing of chronic wounds of the lower extremities in diabetic patients. Phasic electrical stimulation is often employed, which favors nerve activation, and indeed improves nerve function in diabetic rat,106,107 as well as increasing wound healing in diabetic mice108 and man.109 Less is known about the effects of prolonged DC stimulation at a level to mimic naturally occurring wound potentials, although in nondiabetic patients and animals there is usually an improvement in wound tensile strength.54 An in-depth study of diabetic electrophysiology (TEP and wound currents in diabetic skin/cornea) would be beneficial.
Controversies and Alternative Points of View
The simple hypothesis is—wounds generate endogenous EFs, and cells that are responsible for wound healing (and which respond to EFs of physiological magnitude by directional migration) use the endogenous wound EF as a signal to direct them into the wound. Many studies, as mentioned earlier, support this hypothesis. However, not all reports provide consistent support for this theory. For example, Nuccitelli et al.76 found that the human skin wound EF declines with age. This, in general, supports the decreased healing capability of wounds in the older population. Nevertheless, they saw no correlation between the amplitude of the EF and the rate of wound healing. Kucerova et al.110 studied cornea EF and wound healing in Pax6+/−mice that have ocular surface abnormality and disrupted wound healing and corneal epithelial cell migration. They found no correlation between the healing rate and the direction of wound-induced current (wounds with inward currents healed the same as normal wounds with outward currents). Wound healing is an essential mechanism that is conserved in all life forms. Many compensatory mechanisms co-exist to ensure wound healing. One single local factor may contribute to different phase(s) of healing. In Pax6+/− mice, defect of corneal wound healing was significant in the first 2 h after injury, but presumably other mechanisms come into play and the healing in the following hours returns to close to control values.89 Lack of significant correlation of direction of currents to wound healing in the in vivo corneal wound healing also suggests there are compensatory mechanisms. The electric currents with reversed polarity in Pax6+/− mice are less than one-fourth of the magnitude of the control values; thus, its effect on guiding cell migration is likely not enough to counter the effects of other directional cues at the wounds. Further comparative studies are needed to elucidate the relative roles of different directional signals in the various phases of wound healing.
h after injury, but presumably other mechanisms come into play and the healing in the following hours returns to close to control values.89 Lack of significant correlation of direction of currents to wound healing in the in vivo corneal wound healing also suggests there are compensatory mechanisms. The electric currents with reversed polarity in Pax6+/− mice are less than one-fourth of the magnitude of the control values; thus, its effect on guiding cell migration is likely not enough to counter the effects of other directional cues at the wounds. Further comparative studies are needed to elucidate the relative roles of different directional signals in the various phases of wound healing.
Some clinical studies demonstrate efficacy in promoting wound healing using AC or pulsed DC stimulation. This along with the results of Kucerova et al.110 and Sta Iglesia and Vanable14 indicate very interesting, but even less explored, effects on other cell behaviors in addition to directional cell migration. These include cell proliferation, differentiation, and metabolism, among others. These are important cellular responses in wound healing. We would suggest that the significant effects of applied EFs on cell migration over a relatively short period of time (which is experimentally much easier to demonstrate) should not mask other possible cellular responses, which are much less well studied. Technical difficulties limit this type of research. Development of new experimental techniques will allow exploration in this exciting direction and provide significant insights into electrical effects on wound healing not limited to cell migration. Perhaps the presence of the electric signal and not its polarity or direction is important. This would explain why nondirectional (e.g., pulsed AC stimulation where the polarity is switched relatively quickly) can still enhance healing of chronic skin wounds.68,69
Conclusions
Wound EFs and currents appear immediately and spontaneously on injury, rise rapidly, persist for many hours and days during the healing process, and disappear close to the time of complete healing.76 It has been shown that this natural, endogenous wound electric signal is a potent stimulus to cell migration during wound healing, overriding other wound cues such as growth factors, chemicals, and wound void.16 The molecular bases of the generation of these signals are slowly being revealed. For example, in the cornea, chloride (and CLCs) appears to be the dominant ion, and is much more important than sodium.10,81 Extracellular calcium flux at the wound is small,10 but an intracellular calcium wave that passes from cell to cell away from the wound edge is required for normal healing in some systems.89,91 A G protein (guanine nucleotide binding protein alpha subunit)-Ca2+signaling pathway is necessary for actin-dependent wound healing in C. elegansnematode worms,91 but the intracellular calcium release may also activate calcium-activated CLCs.
These results suggest that fluxes of ions are a fundamental component of the wound-healing response. Experimental and clinical manipulation of wound EFs has yielded some promising results for improved wound healing. Accumulating experimental evidences suggest that regulated fluxes of ions and associated electric currents/fields at wounds are an active response of tissues to injury, in the same way that cells and tissues produce growth factors and cytokines in response to injury. Compared with these well-known active biochemical responses to injury and their roles in wound healing, electrical responses of tissues to injury is a new field with many un-answered questions. Further research to elucidate how epithelial tissues regulate ion transport to generate electrical signals that facilitate healing responses may demonstrate an under-studied healing mechanism, and offer new approaches for clinical application of this modality.
Abbreviations and Acronyms
- AC
- alternating current
- [Ca2+]i
- internal calcium concentration
- CLC
- chloride channel
- Cx43
- connexin 43
- DC
- direct current
- EF
- electric field
- ENaC
- epithelial sodium channel
- HCE
- human corneal epithelial
- HVPC
- high-voltage pulsed current
- LFPC
- low-frequency pulsed current
- PC
- pulsed current
- TEP
- trans-epithelial potential
Acknowledgments and Funding Sources
This work was supported by the National Institutes of Health National Eye Institute grant 1R01EY019101, and the Research to Prevent Blindness, Inc.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Dr. Brian Reid is a Project Scientist in the Department of Dermatology, University of California, Davis. His main field of research is using the cornea as a wound-healing model. In particular, he is interested in the mechanisms that produce the natural, endogenous EFs and currents which are generated on corneal injury and which are essential for normal healing. Dr. Min Zhao is a Professor in the Departments of Dermatology and Ophthalmology, University of California, Davis. He is internationally known for the discovery that endogenous electric signals are one of the most important guidance cues directing cell migration and growth to heal wounds. He discovered that PI3 kinase/PTEN molecules are key elements in the electric signaling, the first genes identified in electric signaling in cells. He leads a group studying the molecular and genetic mechanisms underpinning the electric signaling in wound healing and regeneration.

 Wounding tissue (e.g., cornea or skin) initiates generation of large and long-lasting wound electric signals that are essential for normal wound healing.
Wounding tissue (e.g., cornea or skin) initiates generation of large and long-lasting wound electric signals that are essential for normal wound healing.